 About authors:
About authors:
1.Shashi Kant*, Satinder Kumar,
Research scholar department of pharmacy.
2. Dr. Bharat Prashar
HOD & Associate professor
Department of Pharmaceutical Sciences,
Manav Bharti University, Solan (H.P)
shashi_ranaute@yahoo.in
ABSTRACT :
In this review article we discussed about are monospecific identical immune cells that are all clones of a unique parent cell. Monoclonal antibodies have monovalent affinity, in that they bind to the same epitope.Given almost any substance, it is possible to produce monoclonal antibodies that specifically bind to that substance; they can then serve to detect or purify that substance. This has become an important tool in biochemistry, molecular the non-proprietary drug name ends in mabs,in this we discussed type of monoclonal antibodies,preparation,targeting of monoclonal antibodies as well as advantages and disadvantages of monoclonal antibodies. antibodies that are biology and medicine.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1343
INTRODUCTION:-
An antibody is a protein used by the immune system to identify and neutralize foreign objects like bacteria and viruses. Each antibody recognizes a specific antigen unique to its target.
Monoclonal antibodies (mAb) are antibodies that are identical because they were produced by one type of immune cell, all clones of a single parent cell.
Polyclonal antibodies are antibodies that are derived from different cell lines. They differ in amino acid sequence.
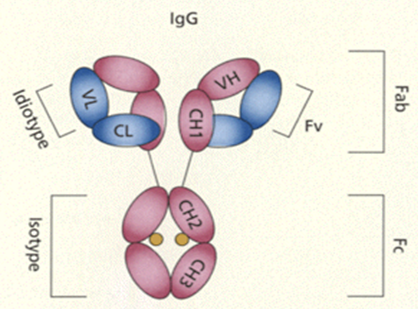
Fig: IgG antibody structure.[2]
Antibodies bind antigen via their variable region (VL and VH), encoded by the V-genes. The CH2 and CH3 domains of heavy chains make up the Fc part and determine the biological activity. N-linked glycosylation sites are indicated in brown. L stands for light chain, H for heavy chain.[1][2][3]
[adsense:468x15:2204050025]
Classes of Igs :
1. IgG: IgG1 (66%), IgG2 (23%), IgG3 (7%) and IgG4 (4%) , blood and tissue liquid
2. IgA: IgA1 (90%) and IgA2 (10%), external secretions (stomach, intestines, saliva, tears, etc.)
3. IgM: 5-10% of total serum Ig [1.5mg/ml serum conc.]
4. IgD: 1% of proteins in the plasma membranes of B-lymphocytes, function unknown [30µg/ml serum conc.] 0.2% of total serum Ig
5. IgE: 0.3µg/ml on the surface of plasma membrane of mast cells, play a role in immediate hypersensitivity and denfense for parasite[5]
History of Monoclonal antibodies development :
Paul Ehrlich at the beginning of the 20th century theorized that a cell under threat grew additional side-chains to bind the toxin, and that these additional side chains broke off to become the antibodies that are circulated through the body. It was these antibodies that Ehrlich first described as "magic bullets" in search of toxins. [3]
Principle :
MYELOMA CELLS have lost the ability to synthesize hypoxanthine-guanine-phosphoribosyl transferase (HGPRT), an enzyme necessary for thesynthesis of nucleic acids through PURINE SALVAGE PATHWAY.
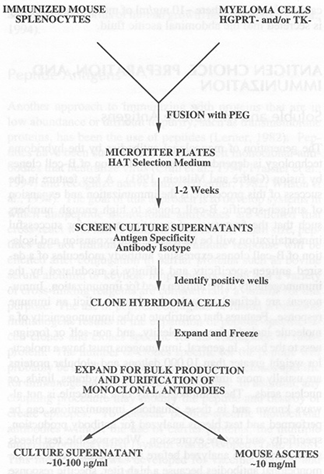
1.The selective culture medium is called HAT medium because it contains HYPOXANTHINE, AMINOPTERIN and THYMIDINE.
2.Unfused myeloma cells cannot grow because they lack HGPRT.
3.Unfused normal spleen cells cannot grow indefinitely because of their limited life span. [6][7][8]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
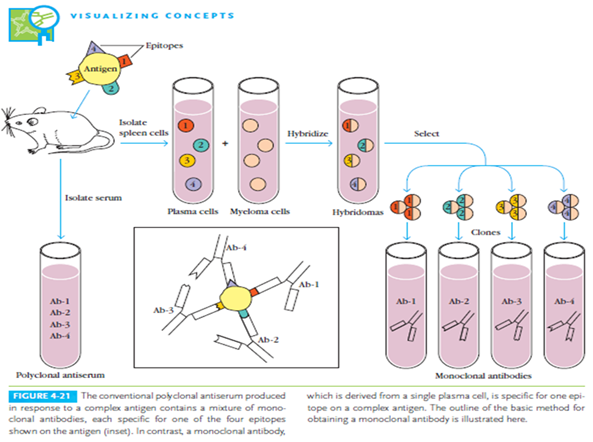
Concept of drug targeting by monoclonal antibody :
Targeting with Antibody depends on the presence of new Antigen from the tumor cells. The antigen associated with tumor cells are called as the” TUMOR MAKER”. Antibodies produced as the results of the specific markers monoclonally can be conjugated with drug molecule which in turn can be targeted to the specific cells or tumor tissues.
Targeting antibodies with drugs involve the following steps:
1. Identification of the antigen produced by the tumor cells.
2. Production of antibody monoclonally against the identified antigen.
3. Formation of drug antibody conjugate or complexes. These complexes concentrate at the tumor site and deliver the drug.
There are several advantages when drugs are delivered as antibody conjugates. The conjugates can specifically reach the target cells without causing any damage to the normal tissue. The drug antibody conjugate could be expected to be the ideal agents for drug targeting in chemotherapy.[8][9]
Types of Monoclonal Antibodies :
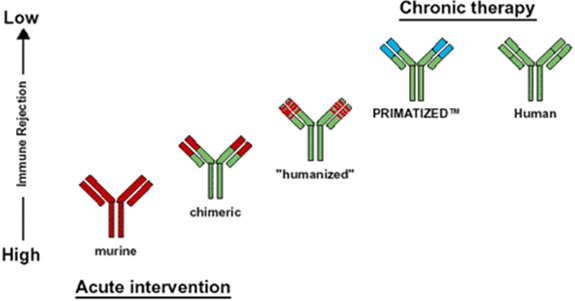
1. Murine monoclonal antibody: Whole antibody is of murine origin produced by Hybridoma technology. Major problems with murine mabs include reduced stimulation of cytotoxicity, formation of complexes after repeated administration, Allergic reactions, Anaphylactic shock. Eg. Aflimomab
2. Chimeric monoclonal antibody: Chimeric antibodies composed of murine variable regions fused onto human constant regions developed by Recombinant DNA technology. Antibodies are Approximately 65% Human origin. This reduces immunogenicity thus increases serum-half life. Eg. Basiliximab, Cetuximab
3. Humanised monoclonal antibody: Humanised antibodies are Produced by grafting murine hypervariable domains into human antibodies Antibodies are approximately 90-95% human origin These bind weakly to the antigens. Eg. Apolizumab, Atlizumab
4. Human monoclonal antibody: Human monoclonal Antibodies are produced By transferring human Immunoglobulin genes Into the murine genome, After which the Transgenic mouse is Vaccinated against the Desired antigen, leading to the production of monoclonal antibodies. Eg. Belimumab, cixutumumab [2][5][10]
Applications of Monoclonal Antibodies :
1. Therapeutic applications : Transplant rejection cardiovascular disease, cancer Infectious diseases Inflammatory diseases
2. Diagnostic applications : Biosensors
3. Clinical applications : purification of drugs, imaging the target.
Monoclonal antibodies for cancer treatment : Monoclonal antibodies act by several mechanisms :-
1. ADCC: Antibody dependent cell-mediated cytotoxicity ADCC Immunoglobulin's clustered on the surface of the targeted cells and exposes its tail {Fc} region, to be recognized by the Fc receptors present on the surface of the macrophages and neutrophils. This causes Lysis of tumor cell.
2. RADIOIMMUNOTHERAPY : It involves the use of radioactively conjugated murine antibodies against cellular antigens. Emitted radiation causes tumor cell lysis. More applicable to lymphomas as they are highly radiosensitive malignancies.
3. ADEPT (Antibody mediated Enzyme prodrug therapy): An antibody developed against a tumor antigen is linked to a drug -activating enzyme and injected to the blood subsequent systemic administration of non-toxic agent or prodrug results in its conversion to a toxic drug & results in cytotoxic effect
4. IMMUNOLIPOSOMES: These are antibody conjugated liposome's Liposome's can carry drugs or therapeutic nucleotides and when conjugated with monoclonal antibodies, deliver the entrapped drug or toxin specially to the target cells.
5. IMMUNOTOXINS: Immunotoxins are proteins that contain a toxin along with an antibody that binds specifically to target cells. All protein toxins are work by enzymatically inhibiting protein synthesis. Various plant & biological toxins have been genetically fused/chemically conjugated with the antibodies that bind to cancer cells.
6. Antibody drug conjugates: Antibody drug conjugates are monoclonal antibodies (mAbs) attached to biologically active drugs by chemical linkers with liable bonds Reduces side effects and show wide therapeutic window Doxorubicin, Duanomycin, Chlorambucil etc. can be conjugated with monoclonal antibodies.
7. Fragments of Mabs: Fragments of Mabs Fab and F( ab ) 2 are less immunogenic but have greater tumor penetration power than normal antibody and are helpful in detecting smaller lesions (<2cm) not seen on computed tomography ScFv are mainly used as delivery vehicles for cancer therapy.[1][3][10][11]
Problems of delivery of monoclonal antibodies :
1. Slow elimination of monoclonal antibodies from the blood, poor vascular permeability, Cross reactivity with normal tissues, Metabolism of monoclonal antibody conjugates.
2. May bind with the targeted epitopes present on other tissues, which may lead to the damage of normal cells.
3. Tumor uptake may be increased through administering high doses. [12]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
New Approaches :
1. Bispecific antibodies: It consists of two antibodies with specificity for distinct antigens and immune effectors. Cell of the 2 paratopes, one can be directed against a tumor Antigen and other against a T-lymphocyte. The Fc region binds to a Fc receptor present on a macrophage. This mechanism cause tumor cell lysis.
2. Antibody + super antigen: In this approach antibody is linked with super antigen. Eg. Staphylococcal enterotoxin A These toxins are binding directly to macrophages and activating them.[ 13]
FDA Approval : The first approved mAbs was OKT-3 [1986], which is a murine IgGa2 protein to deplete T cells in patients with acute rejection of renal allotransplant.
Until Feb 28, 2005, 18 mAbs were approved by FDA, which were applied in the treatment of organ transplant, Cancer, Asthma, Hematopoietic malignancies and psoriasis.
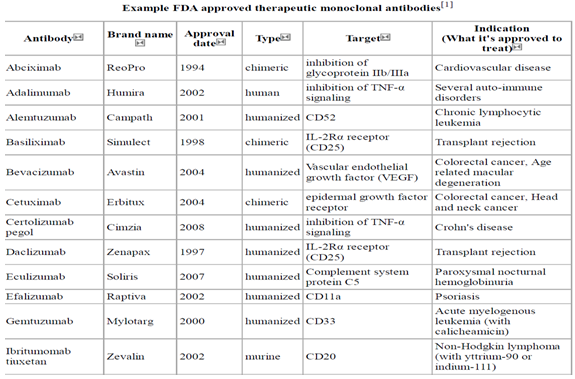
Disadvantages of Monoclonal antibodies :
1. Monoclonal antibodies production, a time consuming process because entire process requires 3-4 months for one fusion experiment.
2. Average affinity of Monoclonal antibodies are generally lower.
3. Any physical/chemical treatment will affect all Monoclonal antibodies in that production. [1][14]
References :
1. Carter P: Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer 2001;1:118-129
2. Dale L Ludwig, et. al. Oncogene(2003) 22, 9097-9106
3. Jancie, M Recheit, etal. Nature biotechnology, 2005, Sep,Vol. 23, No.9Stamatis-Nick C. J Allergy Clin. Immunol, Oct. 2005
4. Schwaber, J; Cohen, EP (1973). "Human x mouse somatic cell hybrid clone secreting immunoglobulins of both parental types". Nature 244 (5416): 444–7.
5. Köhler, G.; Milstein, C. (1975). "Continuous cultures of fused cells secreting antibody of predefined specificity". Nature 256 (5517): 495
6. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (1996) Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention. MMWR Recomm Rep 45: 1–36.
7. Corapi WV, Olsen CW, Scott FW (1992) Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J Virol 66: 6695–6705.
8. Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, et al. (2005) Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: Possible relevance to pathogenesis. J Virol 79: 7819–7826.
9. de Kruif J, Boel E, Logtenberg T (1995) Selection and application of human single chain Fv antibody fragments from a semi-synthetic phage antibody display library with designed CDR3 regions. J Mol Biol 248: 97–105.
10. Kramer RA, Marissen WE, Goudsmit J, Visser TJ, Clijsters-Van der Horst M, et al. (2005) The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur J Immunol 35: 2131–2145.
11. Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22: 27–55.
12. Zwick MB, Wang M, Poignard P, Stiegler G, Katinger H, et al. (2001) Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J Virol 75: 12198–12208.
13. Poon LL, Chan KH, Wong OK, Cheung TK, Ng I, et al. (2004) Detection of SARS coronavirus in patients with severe acute respiratory syndrome by conventional and real-time quantitative reverse transcription-PCR assays. Clin Chem 50: 67–72.
14. La Montagne JR, Taylor SL, Turnbull RJ, the SARS research working group co-chairs (2004) Severe acute respiratory syndrome: Developing a research response. J Infect Dis 189: 642–647.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









