 About Authors:
About Authors:
Dinesh Kumar Jain*, Gajanan Darwhekar, Kapil Mahesh Dutt Swami
College of Pharmacy,
IPS Academy, Rajendra Nagar,
AB Road, Indore.
M.P. India.
*swamikpl@yahoo.com
ABSTRACT
Dandruff and seborrheic dermatitis are the common clinical conditions caused by increased growth of fungi and bacteria on the scalp which results in abnormal proliferation, scaling and flaking of scalp epidermis. In present study an attempt was made to develop hair styling gel of Fluconazole, zinc pyrithione and their combination using different concentration of carbopol 940. The formulations were evaluated and compare for rheology, pH, active content, in vitro drug release, ex vivo drug release, in vitro antidandruff activity and in vivo antidandruff activity. All the formulation are homogenous, transparent and stable but formulation HG1, HG7 and HG11 shows high clarity, optimum rheology, high drug content and drug release, so these three formulations were evaluated for in vitro antidandruff activity. HG11 shows higher zone of inhibition then HG1 and HG7 therefore HG11 was selected for skin irritation test, in vivo study and stability study. HG11 does not show any skin irritation hence it could be an effective formulation for treatment of dandruff and seborrheic dermatitis as compare to other.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1271
INTRODUCTION
Dandruff is a condition in which dead skin cells from the scalp come off in scales that are visible on the hair. The scales are dry, white or greyish and appear as small patches, especially at the top of the head. While it is normal for a small amount of skin cells to flake off due to normal skin growth, dandruff is a special case where an unusually large amount of flaking occurs. This is often accompanied by redness and irritation of the scalp. In some cases, dandruff may be a symptom of a more serious problem, such as seborrheic dermatitis, psoriasis.
There were three factors required for dandruff formation:
1. Natural sebaceous secretions—sebum which is made up of triglycerides and free fatty acids.
2. Malassezia fungi—normal resident flora on human skin and is related to yeast. It sustains itself by feeding on sebum.
3. Individual sensitivity—depends on how genetically susceptible they are to the condition.
The exact mechanism of dandruff formation is now believed to be the result of the formation of enzymes called lipases. The malassezia fungus uses these enzymes to break down sebum to oleic acid. The oleic acid then penetrates the top layer of skin and causes increased skin cell turnover in susceptible people. This in turn causes dandruff flakes and sometimes itching and redness. The current strategy for treating dandruff, namely using some compound to control the growth of malassezia.[1]
Fluconazole is a broad spectrum, antimycotic agent that is active against both Candida albican and Mallesezia furfur and acts by blocking the biosynthesis of ergosterol, the primary sterol derivative of the fungal cell membrane. The zinc pyrithione (ZPTO) heals the scalp by normalizing the epithelial keratinization or sebum production or both. When both drugs are used in combination they show better antidandruff action because of additive effect.
Hair styling gel containing fluconazole and zinc pyrithione will be used to impart wet look, to style the hair and provide antidandruff action. Conventionally available shampoos used against dandruff act for short duration of time, may irritate eye and skin, requires rinsing and washing.[2] Therefore the present formulation provide necessary objective to formulate as Gel.
[adsense:468x15:2204050025]
MATERIALS AND METHODS
Fluconazole was obtained as gift sample from Glenmark Generic Ltd, Verna, Goa. India. Zincpyrithione was procured from Matrix lab. Ltd. Nasik, Maharashtra. India. Carbopol 940, propylene glycol, polyethyleneglycol 400 were purchased fromHiMedia Laboratories Pvt. Ltd., Mumbai. All other chemicals were used of analytical grade.
Formulation of Antidandruff Hairstyling Gel [3]
Carbopol was sprinkled slowly in demeneralised water with constant stirring and kept overnight for hydration. After complete hydration separate solution of methyl paraben and polyethylene glycol-400 was added to the polymeric solution. Solution of drug in propylene glycol was added with stirring to obtained final formulations. Colour and flavour were used for elegance in traces amount. Triethenolamine was used to neutralize the formulation.
Evaluation of Antidandruff Hairstyling gel
Psychorheological Characteristic [4]
Psychorehological Characteristic were studied for colour, clogging, sudden viscosity change and feel properties. The psychorheological characteristics of all formulation is shown in Table 2.
Spreadability [4]
Spreadability is expressed to denote the extent of area to which gel readily spreads on application to skin or affected part. The therapeutic efficacy of a formulation also depends upon its spreading value. Spreadability is expressed in terms of time in seconds taken by two slides to slip off from gel, placed in between the slides under the direction of certain load.
It is calculated by using the formula
S = M. L / T
Where M = weight tied to upper slide, L = length of glass slides, T = time taken to separate the slides
Extrudability study [5]
The extrudability of formulations was determined using aluminium collapsible tubes filled with 10 g gel. Tubes were held between two clamps. A tube was compressed and extrudibility of the formulation was determined in terms of weight in grams required to extrude a 0.5 cm ribbon of gel in 10 s.
Viscosity study [5]
The measurement of viscosity of the prepared gel was done using Brookfield digital Viscometer (Model LV-I+). The viscosity was measured using spindle no. 64 at 10 rpm and 250C. Before measurement deaeration of gel was done and the gel was filled in appropriate wide mouth container. Samples of the gels were allowed to settle over 30 min at the assay temperature (25 ±/10C) before the measurements.
Drug content [4]
The drug content was determined by taking 1 g of gel (equivalent to 20 mg of Fluconazole and 1 mg of ZPTO) in 10 ml volumetric flask diluted with methanol. The above solution was suitably diluted and determined using UV – Vis spectrophotometer at 262 nm for Fluconazole and 245 nm for ZPTO.
Determination of pH [4]
The pH of formulations was determined using digital pH meter. One gram of gel was dissolved in 100 ml of demineralised water and stored for two hours. The measurement of pH of each formulation was done in triplicate. Instrument was calibrated before use with standard buffer solutions at pH 4, 7, and 9.
Turbidimetric measurement
Degree of turbidity of gel was determined by nepheloturbidimeter. Antidandruff hairstyling gel (0.5gm) was added to 150 ml 0.1 N hydrochloric acid under continuous stirring (50 rpm) on magnetic plate at ambient temperature and the increase in turbidity was measured using a turbidimeter.
In vitro release study [6]
The in vitro release study of the prepared gel was carried out using Keshary–Chien (K.C) diffusion cell using a cellophane membrane previously activated in phosphate buffer pH 5.0 for 24 h. The membrane was mounted in K.C cell, kept at 370C. The known quantity of gel (1 g gel containing 20 mg of Fluconazole and 1 mg of ZPTO) was spread uniformly on donor side. Phosphate buffer pH 5.0 was used as the acceptor medium, from which samples were collected at regular intervals for 3 hours and replaced with the same amount of buffer. The drug concentration on the receptor fluid was determined spectrophotometrically in triplicate against appropriate blank.
Ex vivorelease study [6]
The abdominal hair of albino mice, weighing 22-25 g, was shaved using an electric razor after sacrificing with excess chloroform inhalation. The abdominal skin was surgically removed and adhering subcutaneous fat was carefully cleaned. The epidermis was then separated from dermis by soaking the full thickness skin in 2 M sodium bromide solution in water for 6-8 h. The epidermis was thoroughly washed with water, dried at 25% RH, wrapped in aluminium foil and stored in freeze until further use. For ex-vivorelease study, skin was allowed to hydrate for 1 h before being mounted on the Keshary-Chien diffusion cell with the stratum corneum facing the donor compartment. The gel sample was applied on the skin and then fixed in between donor and receptor compartment of Keshary-Chien diffusion cell. The receptor compartment contain phosphate buffer of pH 5.0. The temperature of the medium was thermostatically controlled at 37±10C by surrounding water jacket and the medium was stirred with bar magnet using magnetic stirrer. Aliquots, withdrawn at predetermined intervals of time, were spectrophotometrically estimated at 245 and 262 nm against their respective blank formulation treated in the same manner.
Stability study[4]
The optimized formulation (HG11) was subjected to different temperature condition (80C± 2°C, 25°C ± 2°C, 45°C ± 2°C) and relative humidity 75 ± 5 % RH during stability studies.Formulation was evaluated for transparency, Viscosity, pH and drug content after every 10 days for one month.
Skin irritation test [4]
Three healthy male rats, weighed 200-250 g were selected for the study. An area of 1 cm2 was shaved for each rat to expose sufficient test area. The rats were divided in three group control, standard and test. Formulation HG 11 was applied on the exposed area of rat labelled test whereas drug solution was applied to standard and control were untreated. The test sites were visually observed for erythema and edema daily upto seven days and compared.
In vitro Antidandruff activity [7]
This activity was determined by Sabouraud dextrose diffusion test employing “cup plate technique” using previously sterilized petridish. Solution of gel formulations HG1, HG7 and HG11 and marketed formulation (as a standard) 1 mg/ml was poured into cups bored of size 8mm in sabouraud dextrose plate previously seeded with test organism (Candida albicans). After allowing diffusion of solution for 2 hrs, the plates were incubated at 270C for 48 h. The zone of inhibition measured around each cup was compared with that of the standard.
In vivo Antidandruff activity [8]
In vivoAntidandruff activity was performed on adult male rabbit weighing 2 to 2.5 kg. Hairs were removed from their flanks with electrical clipper and the area of skin (20mm diameter) was scarified with coarse sandpaper. Scarified skin was infected with few drops of culture of Candida albicans. The fungal infection was induced on the rabbit for first 3 days. On the 4th day, treatment was initiated by topical application to the infected sites with gel formulation for another 4 days. The infected skin was analysed for flakes and inflammation.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULT AND DISCUSSION
Hair styling gel of fluconazole and zinc pyrithione alone and combination were prepared with an intention of increasing the contact time of drug with the scalp as compared to topical solution and shampoos. Carbopol940 and Carbopol934 in different concentrations were employed for the preparation of hair styling gel as shown in (Table 1).
Table.1 Formulation of antidandruff hair styling gel
|
Ingredients (%w/w) |
HG1 |
HG2 |
HG3 |
HG4 |
HG5 |
HG6 |
HG7 |
HG8 |
HG9 |
HG10 |
HG11 |
HG12 |
HG13 |
HG14 |
|
Fluconazole |
2 |
2 |
2 |
2 |
2 |
2 |
- |
- |
- |
- |
2 |
2 |
2 |
2 |
|
Zinc pyrithione |
- |
- |
- |
- |
- |
- |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
|
Carbopol 940 |
0.5 |
1 |
1.5 |
2 |
- |
- |
0.5 |
1 |
1.5 |
2 |
0.5 |
1 |
1.5 |
2 |
|
Carbopol 934 |
- |
- |
- |
- |
0.5 |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
|
Propylene glycol |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
|
PEG 400 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Glycerine |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
|
Methyl paraben |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
|
Colour |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Triethanolamine |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
|
D.M. Water |
59.2 |
58.7 |
58.2 |
57.7 |
59.2 |
58.7 |
61.1 |
60.6 |
60.1 |
59.6 |
59.1 |
58.6 |
58.1 |
57.6 |
All the formulations show excellent psycorheological characteristics (Table 2) except HG5 and HG6 prepared using Carbopol 934. Spreadability study shows high spreadability for HG1, HG7 and HG11 than others. Also viscosity study shows that viscosity of hair gel increases with increase in concentration of Carbopol 940. HG1, HG7 and HG11 show viscosity about 34000 cps which was close to marketed preparation. Viscosity of HG5 and HG6 formulated using increasing concentration of Carbopol 934, i.e 0.5 and 1 % w/w respectively was very high (about 55000 cps) (Table 3).This also corresponds to there low spreadability. Due to above results Carbopol 934 was rejected and Carbopol 940 was used for hair gel preparation.
Table 2. Results of Psycorheological characteristics
|
Sr. No |
Formulations |
Colour |
Clogging |
Homogenity |
Texture |
|
1. |
HG1 |
Clear |
No Change |
Excellent |
Smooth |
|
2. |
HG2 |
Clear |
No Change |
Excellent |
Smooth |
|
3. |
HG3 |
Clear |
No Change |
Excellent |
Smooth |
|
4. |
HG4 |
Clear |
No Change |
Excellent |
Smooth |
|
5. |
HG5 |
Turbid |
Change |
Poor |
Smooth |
|
6. |
HG6 |
Turbid |
Change |
Poor |
Smooth |
|
7. |
HG7 |
Clear |
No Change |
Excellent |
Smooth |
|
8. |
HG8 |
Clear |
No Change |
Excellent |
Smooth |
|
9. |
HG9 |
Clear |
No Change |
Excellent |
Smooth |
|
10. |
HG10 |
Clear |
No Change |
Excellent |
Smooth |
|
11. |
HG11 |
Clear |
No Change |
Excellent |
Smooth |
|
12. |
HG12 |
Clear |
No Change |
Excellent |
Smooth |
|
13. |
HG13 |
Clear |
No Change |
Excellent |
Smooth |
|
14. |
HG14 |
Clear |
No Change |
Excellent |
Smooth |
Table 3. Results of various evaluation parameter for antidandruff hair styling gel
|
S.No. |
Formulation |
Spreadability |
Viscocity |
Extrudability |
pH |
Degree of turbidity |
Drug content |
|
1 |
HG1 |
29.88±1.4 |
34360 ±2.54 |
+++ |
7.2 |
12.2±0.21 |
99.3±0.20 |
|
2 |
HG2 |
22.28±0.87 |
38640 ±1.92 |
+++ |
6.8 |
16.8±0.32 |
98.5±0.52 |
|
3 |
HG3 |
15.57±0.68 |
44430 ±2.32 |
+++ |
6.5 |
21.5±0.31 |
96.7±0.41 |
|
4 |
HG4 |
9.3±0.25 |
50800 ±3.43 |
++ |
6.2 |
26.2±0.13 |
92.6±0.45 |
|
5 |
HG5 |
6.6±0.25 |
54600 ±2.55 |
+++ |
7.1 |
47.1±0.33 |
-- -- |
|
6 |
HG6 |
6.5±0.20 |
59800 ±3.12 |
+++ |
6.7 |
46.7±0.24 |
-- -- |
|
7 |
HG7 |
27.36±0.71 |
34180 ±3.34 |
+++ |
7.1 |
12.1±0.37 |
100.03±0.35 |
|
8 |
HG8 |
22.86±0.47 |
38400 ±3.21 |
++ |
6.8 |
16.8±0.23 |
98.3±0.17 |
|
9 |
HG9 |
16.11±0.17 |
44120 ±1.86 |
+++ |
6.4 |
22.4±0.24 |
95.7±0.37 |
|
10 |
HG10 |
9.92±0.14 |
49700 ±2.33 |
++ |
6.1 |
26.1±0.34 |
93.2±0.35 |
|
11 |
HG11 |
27.9±0.74 |
34010 ±2.51 |
+++ |
7.2 |
12.7±0.32 |
98.1±0.32(f) 99.5±0.36(z) |
|
12 |
HG12 |
24.41±0.52 |
38340 ±2.12 |
+++ |
6.9 |
16.9±0.22 |
97.2 ±0.22(f) 98.8±0.24(z) |
|
13 |
HG13 |
15.84±0.93 |
44100 ±1.98 |
++ |
6.5 |
21.6±0.29 |
95.3 ±0.38(f) 96.0±0.42(z) |
|
14 |
HG14 |
9.95±0.34 |
48450 ±2.43 |
++ |
6.2 |
26.2±0.27 |
92.4±0.56(f) 93.2±0.34(z) |
|
15 |
Marketed 1 |
28.1±1.15 |
34560 ±3.12 |
+++ |
6.4 |
12.4±0.34 |
-- -- |
|
16 |
Marketed 2 |
31.1±1.14 |
37150 ±3.21 |
+++ |
6.8 |
16.8±0.36 |
-- -- |
+++ = excellent, ++ = good
The pH of all formulations was found in the range of 6.1-7.2 which was compatible with skin pH (Table 3). Turbidimetric evaluation shows low degree of turbidity in HG1, HG7 and HG11 than other formulation. Low turbidity is due to low concentration of carbopol in formulation (Table 3). Drug content of all formulation were found in the range of 92.6-100.03 % w/w (Table 3).
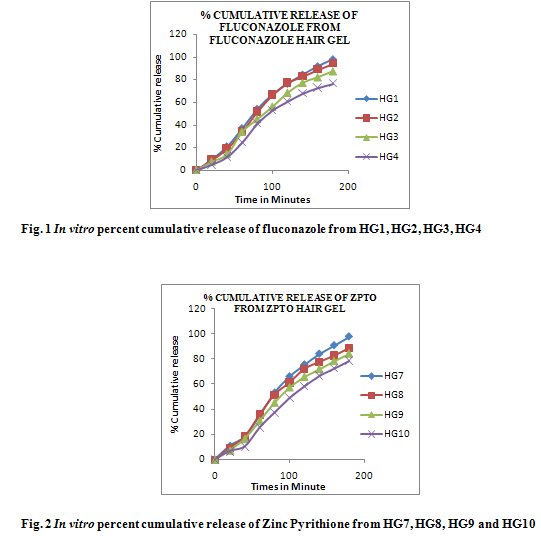
The In vitro release of drug calculated in terms of percent cumulative drug released (Fig 1, 2, 3) shows that drug release from HG1, HG7 and HG11 was significantly greater than HG4, HG10 and HG14. HG1, HG7 and HG11 showed release of about 98-99% after 3 hour which was decreased upto 75-80% in HG4, HG10 and HG14. The decrease in the release could be attributed to increased microviscosity of the gel due to increased polymer concentration which is in agreement with Lauffer’s molecular diffusion theory of polymer gels. The theory states that the diffusion coefficient of a solute is inversely proportional to the volume fraction occupied by the gel-forming agent.
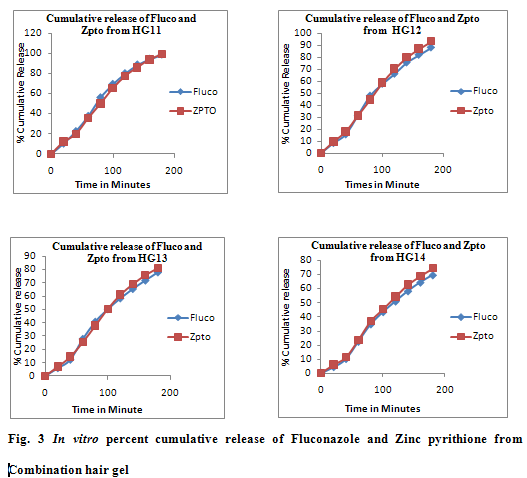
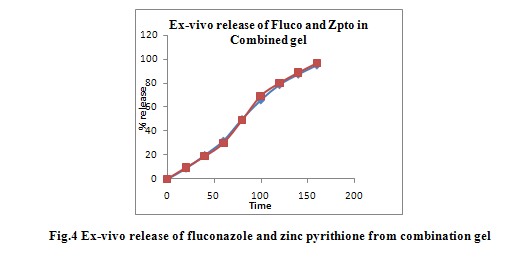
Ex vivorelease studied on optimized HG11 formulation showed the same pattern as that of the in vitro release profile across the cellophane membrane (Fig 4).The selective gel HG11 was taken for stability study at 0-80C (refrigerator), ambient temperature (R.T.) and 45±20C at 75±50C RH for 30 days. The results of stability studies are shown in Table 4. No significant change was observed in transparency, viscosity, pH, drug content upon storage time of 1 month.
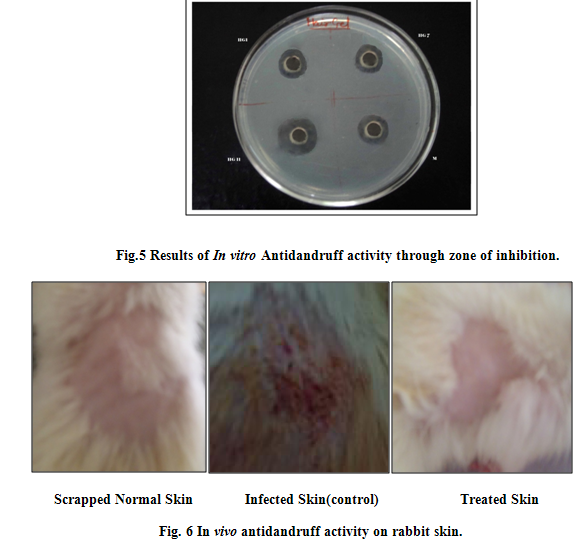
The results of skin irritation test (Table 4) indicate that the prepared gel do not produce any dermatological reaction and were well tolerated by rats. In vitro Antidandruff activity was calculated in terms of zone of inhibition for gels. Formulation HG1, HG7 and HG11 showed 16.15, 14.35 and 25.09 mm inhibition in comparison with marketed formulation with 18.03 mm inhibition respectively (Fig 5). Therefore HG11 formulation was used for in vivo antifungal study which showed no visual flakes and inflammation (Fig.6).
Table 4. Stability study of Hair styling gel
|
Temperature |
0-80c |
Ambient Temprature |
45±20c |
|
Period |
10 days |
20 days |
30 days |
|
Transparency |
Transparent |
Transparent |
Transparent |
|
Viscosity (Cps.) |
34200 |
34080 |
34000 |
|
pH |
7.2 |
7.1 |
7.1 |
|
Drug content |
98.5%(Fluconazole) 99.1%(ZPTO) |
98.2%(Fluconazole) 98.9%(ZPTO) |
98.1%(Fluconazole) 98.9%(ZPTO) |
Table 5 Skin irritation test
|
S.No |
Reaction |
Experimental result |
|
1 |
Redness |
NO |
|
2 |
Edema |
NO |
|
3 |
Inflammation |
NO |
|
4 |
Scar and necrosis |
NO |
CONCLUSION
From the above results it can be concluded that the Hair gel formulation HG11 containing 0.5% Carbopol 940 was suitable for topical application and it shows comparable results with that of marketed product. The formulation HG11 shows superior drug release than others. In carbopol gel formulations, the drug release was decrease with increase in carbopol concentration because polymer concentration increases the viscosity. Viscosity is negatively related to the release of active substance from formulations. Stability studies in all gel formulations showed that, the physical appearance, drug content, pH, rheological properties, and drug release in all gel formulations remain unchanged upon storage for two months. Antimicrobial activity shows that Gel with combination of Fluconazole and Zinc pyrithione shows higher efficacy without any dermal irritancy. Moreover the optimized formulation showed no signs of irritation or inflammation.
REFERENCES
1. Shapiro J., Maddin S. D. Medicated shampoos. Clin dermatol.1996; 14:123-128.
2. Sanfilippo A., Joseph C. An overview of medicated shampoos used in dandruff treatment. P and T2006; 31(7): 396-400.
3. Nayak S.H., Nakhat P.D., Yeole P.G. Development and evaluation of hair styling gel of ketoconazole. Ind. J. Pharm. Sci. 2005; 63(3): 231-233.
4. Kaur LP., Garg R., Gupta G.D. Development and evaluation of topical gel of minoxidil from different polymer bases in application of alopecia. Int. J. Pharm. Sci. 2010; 2(3): 43-47.
5. Chakole C.M., Shende M.A., Khadatkar S.N. Formulation and evaluation of novel combined halobetasol propionate and fusidic acid ointment. Int. J. Chem. Tech. Resear. 2009; 1(1): 103-116.
6. Patel R.P., Patel H.H., Baria A.H. Formulation and evaluation of Carbopol gel containing liposomes of ketoconazole. Int. J. Drug Del. Tech. 2009; 1(2): 42-45.
7. Amelia C., Bulmer S. The antidandruff action of dandruff shampoo. Mycopathologia. 1999; 147(2): 63-65.
8. Saboji J.K., Manvi F.V., Gadad A.P., Patel B.D. Formulation and evaluation of ketoconazole microsponge gel by quassi emulsion solvent diffusion. J. cell and tissue resear. 2011; 11(1): 2691-2696.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









