ABOUT AUTHOR:
Mamta Yadav
M.Pharm
Sagar Institute of Research Technical And Science-pharmacy
Bhopal (M.P)
mamtayadav811@gmail.com
ABSTRACT:
Cancer is the uncontrollable growth of cells. Exponential growth and doubling times of cell is 2 times or 3 times than normal cell growth, doubling times is varies among various kind of tumour’s:
in acute leukemia (2 week)
in multiple myeloma (6 to 12 hour)
Tumour detected when the no. of cells reaches approximately 109 to 1010 cells. Cancer vaccines that boost the immune system’s natural ability to protect body against foreign invaders that causes disease. Vaccines are 2 types: prophylactic and therapeutic. Cancer preventive vaccine target infectious agents that cause cancer and prevent cancer in healthy people and therapeutic vaccines treat cancer in cancer suffering patients.
REFERENCE ID: PHARMATUTOR-ART-1898
Types of cancer vaccines:
1. antigen/adjuvant vaccines,
2. whole cell cancer vaccines,
3. dendritic cell(DC) cell vaccines,
4. viral vector and DNA vaccines,
5. Idioptype vaccines.
In 2006, cancer vaccines approved for use by the U.S. food and drug administration (FDA) known as Gardasil for type 6 and 11 and carvarix, a second HPV vaccine manufactured by Glaxosmithklime for type 16 and 18. Research can use certain immune system cells and their product antibodies created in lab. e.g. – Dendritic cells and costimulatory molecules, idiotype vaccines, anti-id.
Side effects-inflammation, pain swelling, itching, rashes, flu, fever, chill, weakness, dizziness, nausea, vomiting, fatigue, headache, hypersensitivity
INTRODUCTION:
Vaccines boost the immune system’s natural ability to defend the body against infection and to protect it from dangers posed by certain types of damaged or abnormal cells, including cancer cells. Some cancer vaccines, known as cancer preventive vaccines, are designed to prevent cancer from developing in healthy people. Other cancer vaccines, known as cancer treatment vaccines, are intended to treat cancers that have already occurred. The U.S. Food and Drug Administration (FDA) has approved two types of cancer preventive vaccines: A vaccine against the hepatitis B virus, which can cause liver cancer in chronically infected people, and a vaccine against human papilloma virus types 16 and 18, which are responsible for about 70 percent of all cases of cervical cancer. Cancer treatment vaccines are designed to treat cancer by stimulating the immune system to recognize and attack cancer cells. The FDA has not yet approved a cancer treatment vaccine. Effective cancer treatment vaccines are difficult to develop because some cancers can escape detection by the immune system or weaken natural immune responses against cancer cells. Researchers are developing treatment vaccines against many types of cancer and testing them in clinical trials. The side effects of cancer vaccines vary from patient to patient and according to the type of vaccine being used. Most of the side effects reported thus far have been mild and limited to inflammation at the site of the vaccine injection.Traditional vaccines usually contain harmless versions of microbes—killed or weakened microbes, or parts of microbes—that do not cause disease but are able to stimulate an immune response. When the immune system encounters these substances through vaccination, it responds to them, eliminates them from the body, and develops a memory of them. This vaccine-induced memory enables the immune system to act quickly to protect the body if it becomes infected by the same microbe in the future. The immune system’s role in defending against disease-causing microbes has long been recognized. Scientists have also discovered that the immune system can protect the body against threats posed by certain types of damaged, diseased, or abnormal cells, including cancer cells. White blood cells, or leukocytes, play the main role in immune responses. These cells carry out the many tasks required to protect the body against disease-causing microbes and abnormal cells. Some types of leukocytes patrol the body, seeking foreign invaders and diseased, damaged, or dead cells. These white blood cells provide a general—or nonspecific—level of immune protection.
Other types of leukocytes, known as lymphocytes, provide targeted protection against specific threats, whether from a specific microbe or a diseased or abnormal cell. The most important groups of lymphocytes responsible for carrying out immune responses against such threats are B cells and cytotoxic(cell-killing) T cells.
B cells make antibodies, which are large proteins secreted by B cells that bind to, inactivate, and help destroy foreign invaders or abnormal cells. Most preventive vaccines, including those aimed at hepatitis B virus (HBV) and human papilloma virus (HPV), stimulate the production of antibodies that bind to specific, targeted microbes and block their ability to cause infection. Cytotoxic T cells, which are also known as killer T cells, kill infected or abnormal cells by releasing toxic chemicals or by prompting the cells to self-destruct (apoptosis).
Other types of lymphocytes and leukocytes play supporting roles to ensure that B cells and killer T cells do their jobs effectively. Cells that help fine-tune the activities of B cells and killer T cells include helper T cells and dendritic cells, which help activate killer T cells and enable them to recognize specific threats.
Cancer treatment vaccines work by activating B cells and killer T cells and directing them to recognize and act against specific types of cancer. They do this by introducing one or more molecules known as antigens into the body, usually by injection. An antigen is a substance that stimulates a specific immune response. An antigen can be a protein or another type of molecule found on the surface of or inside a cell.
Microbes carry antigens that “tell” the immune system they are foreign—or
“non-self”—and, therefore, represent a potential threat that should be destroyed. In contrast, normal cells in the body have antigens that identify them as “self.” Self antigens tell the immune system that normal cells are not a threat and should be ignored.
Cancer cells can carry both types of antigens. They have self antigens, which they share in common with normal cells, but they may also have antigens that are unique to cancer cells. These cancer-associated antigens mark cancer cells as abnormal, or non-self, and can cause B cells and killer T cells to mount an attack against the cancer.
Cancer cells may also make much larger than normal amounts of certain self antigens. These overly abundant self antigens may be viewed by the immune system as being foreign and, therefore, may trigger an immune response against the cancer. Cancer vaccines are medicines that belong to a class of substances known as biological response modifiers. Biological response modifiers work by stimulating or restoring the immune system’s ability to fight infections and disease. There are two broad types of cancer vaccines:
Preventive (or prophylactic) vaccines, which are intended to prevent cancer from developing in healthy people; and Treatment (or therapeutic) vaccines, which are intended to treat already existing cancers by strengthening the body's natural defenses against cancer.Two types of cancer preventive vaccines have been successfully developed and are available in the United States. However, cancer treatment vaccines remain an experimental form of therapy.
Vaccine
Vaccines for cancer represent an alternative approach to the use of therapeutics. In contrast to traditional vaccines that prevent disease, cancer vaccines enlist the patient’s immune system to destroy existing cancer cells. While simple in concept, the development of products has proven difficult. Problems lie in eliciting sufficient, tumor-selective stimulation of an immune system that is already tolerant of cancer cells. Commercially sponsored cancer vaccines first entered clinical studies in the early-1980s and so companies had at least some experience in the area by 1990.
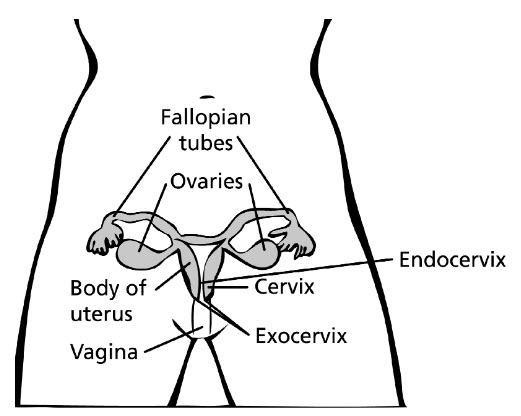
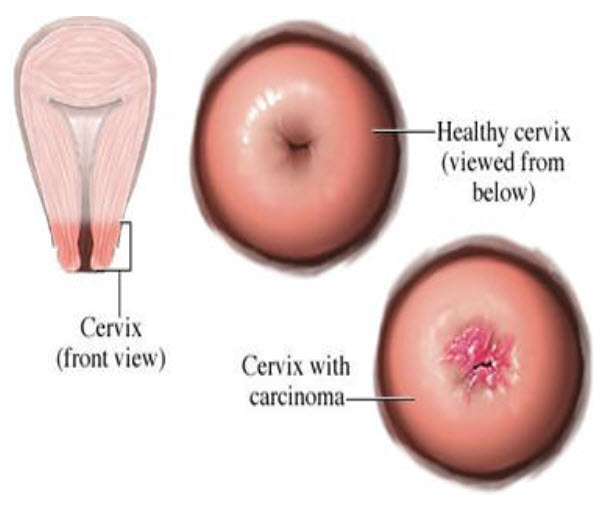
Cervical cancer occurs when abnormal cells on the cervix grow out of control. The cervix is the lower part of the uterus that opens into the vagina. Cervical cancer can often be successfully treated when it's found early. It is usually found at a very early stage through a Pap test.
EVOLUTION
In 1975, virologist Harald zur Hausen presented provocative evidence that HPV, a common infection spread through skin to skin contact and sex that was believed to lead to serious disease only rarely, could cause cervical cancer. Zur Hausen, who for 20 years headed the German Cancer Research Center in Heidelberg, led a team that by the early 1980s had isolated several genotypes of the virus, some of which they linked to genital warts and others to cervical cancer. “For quite a while, we faced a lot of resistance,” says zur Hausen, now a professsor emeritus. But as the polymerase chain reaction assay improved the ability to detect viral DNA, epidemiological data accumulated that backed zur Hausen’s theories. Indeed, one 1999 report found HPV DNA in 99.7% of cervical cancers studied, conclusive evidence that persistent infection with the virus causes the disease. Nearly half a million women worldwide developed cervical cancer in 2002 and it killed 270,000, according to the latest data from the International
Agency for Research on Cancer (IARC). In developed countries, use of the Papanico- laou test, or Pap smear—which swabs the cervix and looks for abnormal cells—has dramatically cut cervical cancer rates over the past 50 years: Only 5000 American women died from the disease in 2002, a 75% drop in mortality since 1950. But much of the world still does not routinely use the Pap smear, making the need for a vaccine that much more pressing. Scientists have identified more than 100 genotypes of HPV, only 40 of which infect the genital tract; of these, about 15 put women at “high risk” for cervical cancer. In the vast majority of cases, the immune system clears HPV infections before they can cause harm.
What causes cervical cancer?
Most cervical cancer is caused by a virus called human papillomavirus, or HPV. You can get HPV by having sexual contact with someone who has it. There are many types of the HPV virus. Not all types of HPV cause cervical cancer. Some of them cause genital warts, but other types may not cause any symptoms.
You can have HPV for years and not know it. It stays in your body and can lead to cervical cancer years after you were infected. This is why it is important for you to have regular Pap tests. A Pap test can find changes in cervical cells before they turn into cancer. If you treat these cell changes, you may prevent cervical cancer.
- Smoking
- Immunosuppression
- Chlamydia infection
- Intrauterine device use
- Oral contraceptives (birth control pills)
- Young age at the first full-term pregnancy
- Multiple full-term pregnancies
- Diethylstilbestrol (DES)
- Family history of cervical cancer
- Diet
- Sexual intercourse at early age
- Multiple sex partners
- Genital infection e.g. herpes simplex virus type 11, human papilloma virus (HPV)
- Early pregnancy with a first baby before 20 years of age.
- Age 40 – 60 years
- Symptoms of established or invasive cancer of the cervix
- Bleeding after sexual intercourse (Postcoital staining)
- Irregular vaginal bleeding
- Vaginal bleeding one year or more after the menopause (Post-menopausal bleeding)
- Foul-smelling vaginal discharge
- Pre-cancer of the cervix is without symptoms.
Estimated new cases and deaths from cervical (uterine cervix) cancer in the United States in 2010:
- New cases: 12,200
- Deaths: 4,210
What are the symptoms?
Abnormal cervical cell changes rarely cause symptoms. But you may have symptoms if those cell changes grow into cervical cancer. Symptoms of cervical cancer may include:
- Bleeding from the vagina that is not normal or a change in your menstrual cycle that you can't explain.
- Bleeding when something comes in contact with your cervix, such as during sex or when you put in a diaphragm.
- Pain during sex.
- Vaginal discharge that is tinged with blood.
How is cervical cancer diagnosed?
As part of your regular pelvic exam, you should have a Pap test. During a Pap test, the doctor scrapes a small sample of cells from the surface of the cervix to look for cell changes. If a Pap test shows abnormal cell changes, your doctor may do other tests to look for precancerous or cancer cells on your cervix.
Your doctor may also do a Pap test and take a sample of tissue (biopsy) if you have symptoms of cervical cancer, such as bleeding after sex.
Making your Pap tests more accurate
Pap test as accurate as possible:
• The best time is at least 5 days after your menstrual period stops.
•Do not douche for 48 hours before the test.
•Do not have sexual intercourse for 48 hours before the test.
•Do not douche or use tampons, birth control foams, jellies, or other vaginal creams, moisturizers or lubricants, or vaginal medicines for 48 hours before the test.
Pelvic exam versus Pap test
Many people confuse pelvic exams with Pap tests. The pelvic exam is a routine part of a woman's health care. During a pelvic exam, the doctor looks at the vulva, vagina, and cervix and feels the reproductive organs, including the cervix, uterus and the ovaries and may do tests for sexually transmitted diseases Pap tests are often done during pelvic exams, but you can have a pelvic exam without having a Pap test. A pelvic exam without a Pap test will not help find abnormal cells of the cervix or cervical cancer at an early stage. The Pap test is often done at the start of the pelvic exam, after the speculum is placed. To do a Pap test, the doctor removes cells from the cervix by gently scraping or brushing it with a special instrument. Pelvic exams may help find other types of cancers and reproductive problems, but a Pap test is needed to find early cervical cancer or pre-cancers.
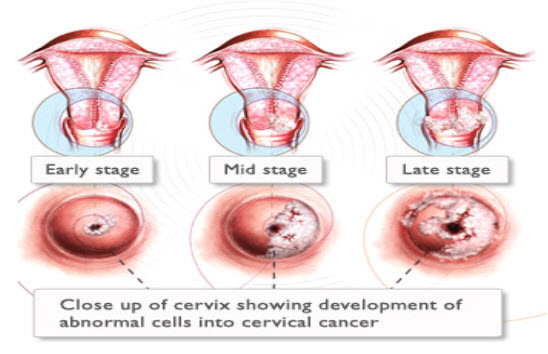
Signs and symptoms of cervical cancer:-
Women with early cervical cancers and pre-cancers usually have no symptoms. Symptoms often do not begin until the cancer becomes invasive and grows into nearby tissue. When this happens, the most common symptoms are:
•Abnormal vaginal bleeding, such as bleeding after vaginal intercourse, bleeding after menopause, bleeding and spotting between periods, and having (menstrual) periods thatare longer or heavier than usual. Bleeding after douching or after a pelvic exam may also occur.
•An unusual discharge from the vagina − the discharge may contain some blood and may occur between your periods or after menopause.
•Pain during intercourse. These signs and symptoms can also be caused by conditions other than cervical cancer. For example, an infection can cause pain or bleeding. Still, if you have any of these signs or other suspicious symptoms, you should see your health care professional right away Ignoring symptoms may allow the cancer to progress to a more advanced stage and lower your chance for effective treatment. Even better, don't wait for symptoms to appear. Have regular Pap tests and pelvic exams.Your primary doctor can often treat pre-cancers and can often perform the colposcopy and biopsy to diagnose pre-cancers and cancers. If there is a diagnosis of invasive cancer, your doctor should refer you to a gynecologic oncologist, a doctor who specializes in cancers of women's reproductive systems.
How is it treated?
The treatment for most stages of cervical cancer includes:
- Surgery, such as a hysterectomy and removal of pelvic lymph nodes with or without removal of both ovaries and fallopian tubes.
- Chemotherapy.
- Radiation therapy.
Depending on how much the cancer has grown, you may have one or more treatments. And you may have a combination of treatments. If you have a hysterectomy, you won't be able to have children. But a hysterectomy isn't always needed, especially when cancer is found very early. It's common to feel scared, sad, or angry after finding out that you have cervical cancer. Talking to others who have had the disease may help you feel better. Ask your doctor about support groups in your area. You can also find people online who will share their experiences with you.
Can cervical cancer be prevented?
The Pap test is the best way to find cervical cell changes that can lead to cervical cancer. Regular Pap tests almost always show these cell changes before they turn into cancer. It's important to follow up with your doctor after any abnormal Pap test result so you can treat abnormal cell changes. This may help prevent cervical cancer.
If you are age 26 or younger, you can get the HPV vaccine, which protects against two types of HPV that cause cervical cancer.
The virus that causes cervical cancer is spread through sexual contact. The best way to avoid getting a sexually transmitted infection is to not have sex. If you do have sex, practice safer sex, such as using condoms and limiting the number of sex partners you have.
Different test for cervical cancer
If a biopsy shows that cancer is present, your doctor may order certain tests to see how far the cancer has spread. Many of the tests described below are not necessary for every patient. Decisions about using these tests are based on the results of the physical exam and biopsy.
Cystoscopy, proctoscopy, and examination under anesthesia
These are most often done in women who have large tumors. They are not necessary if the cancer is caught early. In cystoscopy a slender tube with a lens and a light is placed into the bladder through the urethra. This lets the doctor check your bladder and urethra to see if cancer is growing into these areas. Biopsy samples can be removed during cystoscopy for pathologic (microscopic) testing. Cystoscopy can be done under a local anesthetic, but some patients may need general anesthesia. Your doctor will let you know what to expect before and after the procedure. Proctoscopy is a visual inspection of the rectum through a lighted tube to check for spread of cervical cancer into your rectum. Your doctor may also do a pelvic exam while you are under anesthesia to find out if the cancer has spread beyond the cervix.
Imaging studies
If your doctor finds that you have cervical cancer, certain imaging studies may be done. These include magnetic resonance imaging (MRI) and computed tomography (CT) scans. These studies can show whether the cancer has spread beyond the cervix.
Chest x-ray: Your chest may be x-rayed to see if cancer has spread to your lungs. This is very unlikely unless the cancer is far advanced. If the results are normal, you probably don’t have cancer in your lungs.
Computed tomography (CT): The CT scan is an x-ray procedure that produces detailed cross-sectional images of your body. Instead of taking one picture, like a conventional x-ray, a CT scanner takes many pictures as it rotates around you. A computer then combines these pictures into an image of a slice of your body (think of a loaf of sliced bread). The machine takes pictures of multiple slices of the part of your body that is being studied. CT scans can help tell if your cancer has spread to the lymph nodes in the abdomen and pelvis. They can also be used to see if the cancer has spread to the liver, lungs, or elsewhere in the body. Before the first set of pictures is taken you may be asked to drink 1 to 2 pints of a contrast liquid. You may also receive an IV (intravenous) line through which a different kind of contrast is injected. This helps better outline structures in your body. The IV contrast can cause your body to feel flushed (a feeling of warmth with some redness of the skin). A few people are allergic to the dye and can get hives. Rarely, more serious reactions, like trouble breathing and low blood pressure, can occur. You can be given medicine to prevent and treat allergic reactions, so be sure to tell your doctor if you have ever had a reaction to contrast material used for x-rays. It is also important to let your doctor know about any other allergies. CT scans take longer than regular x-rays and you will need to lie still on a table while they are being done. Also, you might feel a bit confined by the ring-like equipment you’re in when the pictures are being taken. CT scans are sometimes used to guide a biopsy needle precisely into an area of suspected cancer spread. For this procedure, called a CT-guided needle biopsy, the patient remains on the CT scanning table while a radiologist advances a biopsy needle toward the location of the mass. CT scans are repeated until the doctors are confident that the needle is within the mass. A fine needle biopsy sample (tiny fragment of tissue) or a core needle biopsy sample (a thin cylinder of tissue about ½-inch long and less than 1/8-inch in diameter) is removed and examined under a microscope.
Magnetic resonance imaging (MRI): MRI scans use radio waves and strong magnets instead of x-rays to take pictures. The energy from the radio waves is absorbed and then released in a pattern formed by the type of tissue and by certain diseases. A computer translates the pattern of radio waves given off by the tissues into a very detailed image of parts of the body. Not only does this produce cross sectional slices of the body like a CT scanner, it can also produce slices that are parallel with the length of your body. MRI images are particularly useful in examining pelvic tumors. They are also helpful in detecting cancer that has spread to the brain or spinal cord. A contrast material might be injected into a vein just as with CT scans, but is used less often. MRI scans take longer than CT scans − often up to an hour. Also, you have to be placed inside a tube-like piece of equipment, which is confining and can upset people with claustrophobia (a fear of enclosed spaces). Special, “open” MRI machines that are not so confining may be an option for some patients; the downside of these is that the images may not be as good. The machine also makes a thumping noise that some people find disturbing. Some places provide headphones with music to block this noise out. A mild sedative is helpful for some people.
Intravenous urography: Intravenous urography (also known as intravenous pyelogram or IVP) is an x-ray of the urinary system taken after a special dye is injected into a vein. This dye is removed from the bloodstream by the kidneys and passes through the ureters and into the bladder (the ureters are the tubes that connect the kidneys to the bladder). This test finds abnormalities in the urinary tract, such as changes caused by spread of cervical cancer to the pelvic lymph nodes, which may compress or block a ureter. IVP is rarely used currently to evaluate patients with cervical cancer. You will not usually need an IVP if you have already had a CT or MRI.
Positron emission tomography: Positron emission tomography (PET) uses glucose (a form of sugar) that contains a radioactive atom. Cancer cells in the body absorb large amounts of the radioactive sugar and a special camera can detect the radioactivity. This test can help see if the cancer has spread to lymph nodes. PET scans can also be useful if your doctor thinks the cancer has spread but doesn’t know where. PET scans can be used instead of other types of x-rays because they scan your whole body. Some machines combine a CT scan and a PET scan to even better pinpoint the tumor. This test is rarely used for patients with early cervical cancer, but may be used to look for more advanced disease.
Preventive vaccines work
Target infectious agents that cause or contribute to the development of cancer. They are similar to traditional vaccines, which help prevent infectious diseases such as measles or polio by protecting the body against infection. Both cancer preventive vaccines and traditional vaccines are based on antigens that are carried by the infectious agents and that are relatively easy for the immune system to recognize as foreign.
Vaccines for cancer represent an alternative approach to the use of therapeutics. In contrast to traditional vaccines that prevent disease, cancer vaccines enlist the patient’s immune system to destroy existing cancer cells. While simple in concept, the development of products has proven difficult. Problems lie in eliciting sufficient, tumor-selective stimulation of an immune system that is already tolerant of cancer cells. Commercially sponsored cancer vaccines first entered clinical studies in the early-1980s and so companies had at least some experience in the area by 1990.
Preventive Vaccines Been Approved For Use In The United States:-
In 2006, the U.S. Food and Drug Administration (FDA) approved the vaccine known as Gardasil®, which protects against infection by two types of HPV—specifically, types 16 and 18—that cause approximately 70 percent of all cases of cervical cancer worldwide. At least 17 other types of HPV are responsible for the remaining 30 percent of cervical cancer cases. Gardasil also protects against HPV types 6 and 11, which are responsible for about 90 percent of all cases of genital warts. However, these two HPV types do not cause cervical cancer.
In 2008, the FDA expanded Gardasil’s approval to include its use in the prevention of HPV-associated vulvar and vaginal cancers.
Gardasil, manufactured by Merck & Company, is based on HPV antigens that are proteins. These proteins are used in the laboratory to make four different types of “virus-like particles,” or VLPs, which correspond to HPV types 6, 11, 16, and 18. The four types of VLPs are then combined to make the vaccine. Because Gardasil targets four HPV types, it is called a quadrivalent vaccine. In contrast with traditional vaccines, which are often composed of weakened, whole microbes, the VLPs in Gardasil are not infectious. However, they are still able to stimulate the production of antibodies against HPV types 6, 11, 16, and 18.
A second HPV vaccine manufactured by GlaxoSmithKline and known by the name Carvarix® has also been developed. Although Carvarix has been approved for use in Europe, it has not yet been approved by the FDA for use in the United States. In contrast with Gardasil, Carvarix is a bivalent vaccine. It is composed of VLPs made with proteins from HPV types 16 and 18. Therefore, it provides protection only against these two HPV types.
The public health benefits of vaccines against HPV types 16 and 18 may extend beyond reducing the risks of cervical cancer, vaginal cancer, and vulvar cancer. Evidence suggests that chronic infection by one or both of these virus types is also associated with cancers of the anus, penis, and or pharynx.
The FDA has approved one other type of cancer preventive vaccine, which protects against HBV infection. Chronic HBV infection can lead to liver cancer. The first HBV vaccine was approved in 1981, making it the first cancer preventive vaccine to be successfully developed and marketed. Today, most children in the United States are vaccinated against HBV shortly after birth.
Many scientists believe that microbes cause or contribute to between 15 percent and 25 percent of all cancers diagnosed worldwide each year, with the percentages being lower in developed countries than in developing countries. The International Agency for Research on Cancer (IARC) has classified several microbes as carcinogenic (causing or contributing to the development of cancer in people), including HPV and HBV. These infectious agents—bacteria, viruses, and parasites—and the cancer types with which they are most strongly associated are listed in the table below.
|
Infectious Agents |
Type of |
Associated Cancer(s) |
|
hepatitis B virus (HBV) |
virus |
hepatocellular carcinoma (a type of liver cancer) |
|
hepatitis C virus (HCV) |
Virus |
hepatocellular carcinoma (a type of liver cancer) |
|
human papillomavirus (HPV) types 16 and 18, as well as other HPV types |
Virus |
cervical cancer; vaginal cancer; vulvar cancer; |
|
Epstein-Barr virus |
Virus |
Burkett lymphoma; non-Hodgkin lymphoma; Hodgkin lymphoma; nasopharyngealcarcinoma(cancer of the upper part of the throat behind the nose) |
|
human T-cell lymph tropic virus 1 (HTLV1) |
Virus |
acute T-cell leukemia |
|
Helicobacter pylori |
bacterium |
stomach cancer |
|
schistosomes (Schistosoma hematobium) |
parasite |
bladder cancer |
|
liver flukes (Opisthorchis viverrini) |
Parasite |
cholangio carcinoma (a type of liver cancer) |
Conclution:-
Cancer treatment vaccines are designed to treat cancers that have already occurred. They are intended to delay or stop cancer cell growth; cause tumor shrinkage; prevent cancer from coming back; or eliminate cancer cells that are not killed by other forms of treatment, such as surgery, radiation therapy, or chemotherapy. Most important is the fact that cancer cells carry normal self antigens in addition to any cancer-associated antigens. Furthermore, cancer cells sometimes undergo genetic changes that lead to the loss of cancer-associated antigens. Finally, cancer cells can produce chemical messages that suppress specific anticancer immune responses by killer T cells. As a result, even when the immune system recognizes a growing cancer as a threat, the cancer may still escape a strong attack by the immune system.
The FDA has not approved any type of cancer treatment vaccine. Producing effective treatment vaccines has proved much more difficult and challenging than developing cancer preventive vaccines. Although researchers have identified many cancer-associated antigens, these molecules vary widely in their capacity to stimulate a strong anticancer immune response. Two major areas of research aimed at developing better cancer treatment vaccines involve the discovery of new cancer-associated antigens that may prove more effective in stimulating immune responses than the already known antigens and the development of new methods to enhance the ability of cancer-associated antigens to stimulate the immune system. Research is also under way to determine how to combine multiple antigens within a single cancer treatment vaccine to produce optimal anticancer immune responses. In addition, researchers are trying to identify the mechanisms by which cancer cells evade or suppress anticancer immune responses. Gardasil for type 6 and 11 and carvarix, a second HPV vaccine manufactured by Glaxosmithklime for type 16 and 18. Research can use certain immune system cells and their product antibodies created in lab. e.g. – Dendritic cells and costimulatory molecules, idiotype vaccines, anti-id.
Side effects of vaccine-inflammation, pain swelling, itching, rashes, flu, fever, chill, weakness, dizziness, nausea, vomiting, fatigue, headache, hypersensitivity. In addition, researchers are trying to identify the mechanisms by which cancer cells evade or suppress anticancer immune responses.
KEY FACTS ABOUT CERVICAL CANCER
•Every year more than 270000 women die from cervical cancer, more than 85% of these deaths are in low and middle income countries.
•Cervical cancer is caused by sexually-acquired infection with Human papillomavirus (HPV). Most people are infected with HPV shortly after onset of sexual activity.
•accination against HPV in girls 9 to 13 years old combined with regular screening in women over age 000 new cervical cancer cases diagnosed every year.
•Sur vival rates for cervical cancer can be further improved by establishing effective cancer treatment programmes.
REFRENCES
1.Pardoll DM. Cancer immunology. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG, editors. Abeloff's Clinical Oncology. 4th ed. Philadelphia: Churchill Livingstone, 2008.
2.Murphy KM, Travers P, Walport M, editors. Janeway's Immunobiology. 7th ed. New York: Garland Science, 2007.
3.Waldmann TA. Effective cancer therapy through immunomodulation. Annual Review of Medicine 2006; 57:65–81.
4.Emens LA. Cancer vaccines: On the threshold of success. Expert Opinion on Emerging Drugs 2008; 13(2):295–308.
5.Sioud M. An overview of the immune system and technical advances in tumor antigen discovery and validation. Methods in Molecular Biology 2007; 360:277–318.
6.Pazdur MP, Jones JL. Vaccines: An innovative approach to treating cancer. Journal of Infusion Nursing 2007; 30(3):173–178.
7.Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nature Reviews Cancer 2006; 6(3):204–216.
8.Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: Prospects and challenges for the 21st century. European Journal of Immunology 2007; 37(Suppl 1):S148–S155.
9.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clinical Science 2006; 110(5):525–541.
10.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. Journal of Clinical Investigation 2006; 116(5):1167–1173.
11.Barr E, Sings HL. Prophylactic HPV vaccines: New interventions for cancer control. Vaccine 2008; August 9 [Epub ahead of print].
12.U.S. Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part 1: Immunization of infants, children, and adolescents. Morbidity and Mortality Weekly Report 2005; 54(No. RR–16):1–23.
13.Parkin DM. The global health burden of infection-associated cancers in the year 2002. International Journal of Cancer 2006; 118(12):3030–3044.
14.Mueller NE. Cancers caused by infections: Unequal burdens. Cancer Epidemiology, Biomarkers & Prevention 2003; 12(3):237s.
15.International Agency for Research on Cancer (2008). IARC monographs on the evaluation of carcinogenic risks to humans. Overall evaluations of carcinogenicity to humans: Group 1: Carcinogenic to humans. Retrieved October 3, 2008, from: Rivoltini L, Canese P, Huber V, et al. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: How can we tilt the balance towards immune-mediated cancer control? Expert Opinion on Biological Therapy 2005; 5(4):463–476.
16.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nature Medicine 2004; 10(9):909–915.
17.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunology and Immunotherapy 2001; 50(1):3–15.
18.Parmiani G, Russo V, Marrari A, et al. Universal and stemness-related tumor antigens: Potential use in cancer immunotherapy. Clinical Cancer Research 2007; 13(19):5675–5679.
19.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique tumor antigens: Immunobiology and use in clinical trials. The Journal of Immunology 2007; 178(4):1975–1979.
20.Lollini PL, Forni G. Cancer immunoprevention: Tracking down persistent tumor antigens. Trends in Immunology 2003; 24(2):62–66.
21.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: Moving beyond current paradigms. Clinical Cancer Research 2007; 13(13):3776–3782.
22.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392(6673):245–252.
23.Finn OJ. Cancer immunology. The New England Journal of Medicine 2008; 358(25):2704–2715.
24.Curigliano G, Spitaleri G, Dettori M, et al. Vaccine immunotherapy in breast cancer treatment: Promising, but still early. Expert Review of Anticancer Therapy 2007; 7(9):1225–1241.
25.Tacken PJ, deVries JM, Torensma R, Fidgor CG. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nature Reviews Immunology 2007; 7(10):790–802.
26.Garnett CT, Greiner JW, Tsang K-Y, et al. TRICOM vector based cancer vaccines. Current Pharmaceutical Design 2006; 12(3):351–361.
27.de Cerio AL, Zabalegui N, Rodríguez-Calvillo M, Inoges S, Bendandi M. Anti-idiotype antibodies in cancer treatment. Oncogene 2007; 26(25):3594–3602.
28.Chiarella P, Massi E, De Robertis M, Signori E, Fazio VM. Adjuvants in vaccines and for immunisation: Current trends. Expert Opinion on Biological Therapy 2007; 7(10):1551–1562.
29.Herr HW, Morales A. History of Bacillus Calmette-Guérin and bladder cancer: An immunotherapy success story. The Journal of Urology 2008; 179(1):53–56.
30.Emens LA. Chemotherapy and tumor immunity: An unexpected collaboration. Frontiers in Bioscience 2008; 13:249–257.
31.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298(5594):850–854.
32.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. Journal of Clinical Oncology 2005; 23(10):2346–2357.
33.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314(5796):126–129.
34.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nature Reviews Cancer 2008; 8(4):299–308.
35.Ng LG, Mrass P, Kinjyo I, Reiner SL, Weninger W. Two-photon imaging of effector T-cell behavior: Lessons from a tumor model. Immunological Reviews 2008; 221:147–162.
36.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature Reviews Immunology 2006; 6(4):295–307
37.Adam E, Kaufman RH, Adler-Storthz K, et al. A prospective study of association of herpes simplex virus and human papillomavirus infection with cervical neoplasia in women exposed to diethylstilbestrol in utero. Int J Cancer. 1985; 35(1):19-26.
38.American Cancer Society. Cancer Facts and Figures 2013. Atlanta, Ga: American Cancern Society; 2013. American Cancer Society. Cancer Prevention and Early Detection Facts and Figures 2010.
39.Fyles A, Keane TJ, Barton M, Simm J. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol m1992; 25:273-279.
40. Girinsky T, Rey A, Roche B, et al. Overall treatment time in advanced cervical carcinomas: a critical parameter in treatment outcome. Int J Radiat Oncol Biol Phys 1993; 27:1051-1056.
41. Lanciano RM, Pajak TF, Martz K, Hanks GE. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a patterns-of-care study. Int J Radiat Oncol Biol Phys 1993; 25:391-397.
42.Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys 1995; 32:1275-1288
43.Petereit DG, Sarkaria JN, Chappell R, et al. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys 1995; 32:1301-1307.
44. Eifel PJ, Levenback C, Wharton JT, Oswald MJ. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1995; 32:1289-1300.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











