{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Dilip G. Maheshwari, Rujuta D. Patel*
Department of Quality Assurance,
L.J. Institute of Pharmacy, Sarkhej, Ahemdabad, Gujarat, India
*Rujutapatel09@gmail.com
ABSTRACT:
A new sensitive, simple, rapid and precise spectrophotometric method has been developed for simultaneous estimation of Torsemide and Amiloride HCl in pharmaceutical dosage form. This method was based on UV spectrophotometric determination of two drugs, using absorbance correction method. It involves measurement of absorbances at two wavelengths 288nm(λmax of Torsemide (TOR)) and 361nm (λmax of Amiloride HCl (AMI)) in methanol for the simultaneous quantitative determination of Torsemide and Amiloride HCl in the binary mixture without previous separation. The linearity was observed in the concentration range of 5 -25 μg/ml for Torsemide and 5-25 μg/ml for Amiloride HCl. The accuracy and precision of the method was determined and validated statically. The method showed good reproducibility and recovery with % RSD less than 2. Method was found to be rapid, specific, precise and accurate, can be successfully applied for the routine analysis of Torsemide and Amiloride HCl in combined dosage form without any interference by the excipients. The method was validated according to ICH guidelines.
INTRODUCTION
Torsemide is a pyridine-sulfonyl urea. It is loop diuretic mainly used in the management of edema associated with congestive heart failure and to reduce the swelling and fluid retention caused by various medical problems, including heart or liver disease.(1)Its chemical name is. 1-{4-[(3-methylphenyl)amino]pyridine-3-sulfonyl}-3-(propan-2-yl)urea.(2,9-12) The structure of Torsemide is shown in Fig 1.
Amiloride HCl is an antikaliuretic-diuretic agent, is a pyrazine-carbonyl-guanidine that is unrelated chemically to other known antikaliuretic or diuretic agents. Amiloride is used in conjunction with diuretics to spare potassium loss. It is the salt of a moderately strong base (pKa 8.7).(3) It is designated chemically as 3,5-diamino-6-chloro-N-(diaminomethylene) pyrazine -2-carboxamide monohydrochloride, dehydrate.(4)The structure of AmilorideHCl is shown in Fig 2.
Torsemide has been marketed in combination with Amiloride HCl in tablets for the treatment of essential hypertension. Torsemide and Amiloride HCl are used combine in the treatment for management of primary hypertension and edema in congestive heart failure.
Literature survey revealed that, Torsemide is official in United States Pharmacopoeia(5); Amiloride HCl is official in Indian Pharmacopoeia and United States Pharmacopoeia(6,7). However, the combination is not official in any pharmacopoeia. On detailed literature survey, it was found that through individually these drugs have been analyzed by many methods, but there is no method available for the estimation of Torsemide and Amiloride HCl drugs in the combine dosage form. Therefore, it was thought worthwhile to develop simple, precise, accurate UV spectrophotometric method for simultaneous determination of Torsemide and Amiloride HCl in tablets.The proposed method was applied to the determination of analyts in pharmaceutical preparations, with satisfactory result. Validation was done with respect to various parameters, as per ICH guideline.
MATERIAL AND METHODS
Instrumentation
A UV-visible spectrophotometer, model UV 1800 (Shimadzu) was used to measure absorbance of the resulting solutions using UV-Probe software version 2.31. A Digital analytical balance (Wenstar DA13-220) and ultrasonic sonicator (Equitron) were used in the study.
Reagents and materials
Pure Torsemide (TOR) & Amiloride HCl (AMI) kindly gifted as a gift sample by Intas (astron) pharma, Ahemdabad, India and Vapi care pharma, Vapi, India. TORSINEX-A Tablet formulation procured from local market. All analytical grade chemicals and solvents were obtained from Merck (India). Methanol used as solvent in the study. Borosil volumetric flasks of 10, 50,100 ml capacity and pipettes – 1ml, 5ml,10ml, beakers, measuring cylinders etc.
PREPARATION OF SOLUTIONS
Preparation of Standard Stock Solutions
Torsemide (1000 μg/ml)
Accurately weighed TOR (100 mg) was transferred to a 100 ml volumetric flask, dissolved in methanol and diluted to the mark with same solvent to obtain a standard stock solution (1000 μg/ml).
AmilorideHcl (1000 μg/ml)
Accurately weighed AMI (100 mg) was transferred to a 100 ml volumetric flask, dissolved in methanol and diluted to the mark with same solvent to obtain a standard stock solution (1000 μg/ml).
Preparation of Working Standard Solutions
Torsemide (100 μg/ml)
Standard Stock solution (5 ml) was transferred to a 50 ml volumetric flask and diluted up to the mark with methanol.
Amiloride HCl (100 μg/ml)
Standard Stock solution (5 ml) was transferred to a 50 ml volumetric flask and diluted up to the mark with methanol.
Preparation of calibration curve
Calibration curve for Torsemide
Aliquots of working standard solution of TOR (100 μg/ml) 0.5, 1, 1.5, 2 and 2.5ml were transferred into a series of 10 ml volumetric flasks and volume was adjusted to the mark with methanol to get concentrations 5, 10, 15 20 and 25 μg/ml. Absorbance of each solution was measured at 288 nm using methanol as a blank. Calibration curve was obtained by plotting respective absorbance against concentration in μg/ml and the regression equation was computed.
Calibration curve for Amiloride HCl
Aliquots of working standard solution of AMI (100 μg/ml) 0.5, 1, 1.5, 2 and 2.5ml were transferred into a series of 10 ml volumetric flasks and volume was adjusted to the mark with methanol to get concentrations 5, 10, 15 20 and 25 μg/ml. Absorbance of each solution was measured at 288 nm and 361 nm using methanol as a blank. Calibration curve was obtained by plotting respective absorbance against concentration in μg/ml and the regression equation was computed.
Application of absorption correction method
Two wavelengths from spectra, 288nm (TOR) and 361nm(AMI) were selected. The absorptivity values of TOR and AMI were determined at selected wavelengths. At 361 nm,estimation of AMI was done where no interference of TOR. The absorbance of working standard solution of AMI at 361 nm was calculated using equation (1) and then from the total absorbance of sample mixture at wavelength 288 nm, the contribution due to AMI was subtracted. The calculated absorbance was called as corrected absorbance for TOR. The concentrations of TOR (Cy) at 288 nm using the corrected absorbance was determine using absorptivity value.The concentration of two drugs in the mixture can be calculate using following equations,
CX= A (361 nm)/ (a(1%,1cm)361 nm)……(1)
AY(288 nm) = CX×(a(1%,1cm)288 nm)……(2)
CAx= A(288 nm) – AY………..(3)
CY = CAx/ a(1%,1cm)288 nm…..(4)
Where,
Cx = Concentration of Amiloride HCl
Cy = Concentration of Torsemide
A288 nm = Absorbance of mixture at 288 nm
A361 nm= Absorbance of mixture at 361 nm
CAx288 nm= Corrected absorbance of Torsemide at288 nm
Ay288 nm = Absorbance of Amiloride HCl at 288nm.
Preparation of sample solution
For the estimation of the drug in tablet formulation twenty tablets were weighed and their average weight was determined. The tablets were then finely powdered. Appropriate quantity equivalent to 10 mg TOR and 5 mg AMI was accurately weighed. The powder was transferred to 100 ml volumetric flask and shaken vigorously with methanol for 15 min and the solution was sonicated for 15 minutes and filtered through Whatman filter paper No. 41. Necessary dilutions are made with methanol to give final concentration 20μg/ml of TOR and10 μg/mlAMI respectively. The absorbance’s values were read at 288 and 361 nm and concentration was obtained by solving the absorption correction equations.
Method Validation(8)
The developed method was validated with respect to linearity, accuracy, intraday and interday precision, limit of detection (LOD) and limit of quantitation (LOQ) and robustness in accordance with the ICH guideline.
Linearity and Range
Linearity was studied by preparing standard solutions at 5 different concentrations. The linearity range for Torsemide and Amiloride HCl were found to be 5-25 μg/ml and 5- 25 μg/ml respectively. Linearity was assessed in the terms of slope, intercept and correlation coefficient for both the drugs.
Precision
The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision may be considered at three levels: repeatability, intermediate (intraday) precision and reproducibility (interday precision).
1) Intraday Precision
Solutions containing 10, 15, 20 μg/ml of TOR and 10, 15, 20 μg/ml of AMI were analysed three times on the same day and %R.S.D was calculated.
2) Interday Precision
Solutions containing 10, 15, 20 μg/ml of TOR and 10, 15, 20 μg/ml of AMI were analyzed on three different successive days and %R.S.D was calculated.
3) Repeatability
Method precision of experiment was performed by preparing the standard solution of TOR (15 μg/ml) and AMI (15 μg/ml) for six times and analysed as per the proposed method. Coefficient of variation (%CV) was not more than 2%.
Accuracy
Accuracy expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found. Recovery studies were carried out by addition of standard drug to the sample at 3 different concentration levels (80%, 100%, 120%) taking into consideration percentage recovery of added bulk drug samples. The experiment was repeated three times by spiking previously analysed samples of tablet with three different concentrations of standards.
Limit of Detection (LOD)
Limit of detection can be calculated using following equation as per ICH guidelines.
LOD = 3.3 x (N / S)
Where, N is the standard deviation of the peak areas of the drug and S is the slope of the corresponding calibration curve.
Limit of Quantification (LOQ)
Limit of quantification can be calculated using following equation as per ICH guidelines.
LOQ = 10 x (N / S)
Where, N is the standard deviation of the peak areas of the drug and S is the slope of the corresponding calibration curve.
RESULT & DISCUSSION
Selection of wavelength for simultaneous estimation of TOR and AMI
To determine the wavelength for measurement, TOR (20 μg/ml) and AMI (10 μg/ml) solutions were scanned between 400-200 nm. Absorbance maxima were obtained at their λmax 288 nm and 361 nm for TOR and AMI respectively.
METHOD VALIDATION
Linearity & Range
The linearity of TOR and AMI was found to be in the range of 5-25 μg/ml and 5-25 μg/ml respectively. Linearity data for TOR and AMI are depicted in Table 1. Calibration curve of TOR and AMI are shown in Figure 7, 8 and 9 respectively. Linear regression equations and correlation-coefficient values for TOR and AMI are shown in Table 2.
Precision
1. Intraday Precision
The data for Intraday precision for TOR and AMI is in range of % RSD was found to be 0.10 – 0.9 % for TOR at 288 nm and 0.16- 0.54 % and 0.15 – 0.79 % for AMI at 288 nm and 361 nm respectively.
2. Interday Precision
The data for Intraday precision for TOR and AMI is in range of % RSD was found to be 0.47 -2.0 % for TOR at 288 nm and 0.16-0.77 % and 0.10- 1.6% for AMI at 288 nm and 361 nm respectively.
3. Repeatability
The data for repeatability for TOR and AMI was found to be 1.72 % for TOR at 288nm and 0.76%, 1.0% for AMI at 288 nm and 361nm respectively.
Accuracy
Accuracy of the method was confirmed by recovery study from marketed formulation at three levels (80%, 100%, and 120%) of standard addition. Percentage recovery for TOR and AMI were found to be in the range of 98.48-101.08% and 100.16-101.8%. Data indicating recovery studies of Torsemide and Amiloride HCl shown in Table 3.
LOD and LOQ
LOD values for TOR and AMI were found to be 1.85μg/ml and 1.44μg/ml respectively. LOQ values for TOR and AMI were found to be 5.6μg/ml and 4.36μg/ml respectively.
Analysis of Marketed formulation
Applicability of the proposed method was tested by analyzing the commercially available tablet formulation. (TORSINEX-A). It is shown in Table:4
CONCLUSION
A simple, rapid, accurate and precise spectrophotometric method has been developed and validated for the routine analysis of torsemide and amilorideHCl in API and tablet dosage forms. The spectrophotometric method is suitable for the simultaneous determination of Torsemide and Amiloride HCl in multi-component formulations without interference of each other. The absorbance correction method is rapid, simple and sensitive. The developed method is recommended for routine and quality control analysis of the investigated drugs in two component pharmaceutical preparations.
ACKNOWLEDGEMENTS
The authors are thankful to Vapi care pharma, Vapi, Gujarat and Intas pharmaceuticals(Astron), Ahemdabad, Gujrat for providing drug samples to carry out this work and DR. Kilambi Pundrikakshudu for providing moral support from L.J institute of pharmacy.
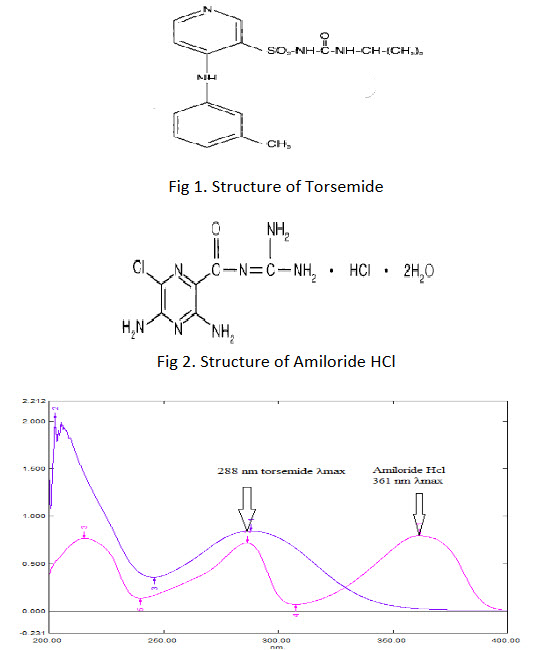
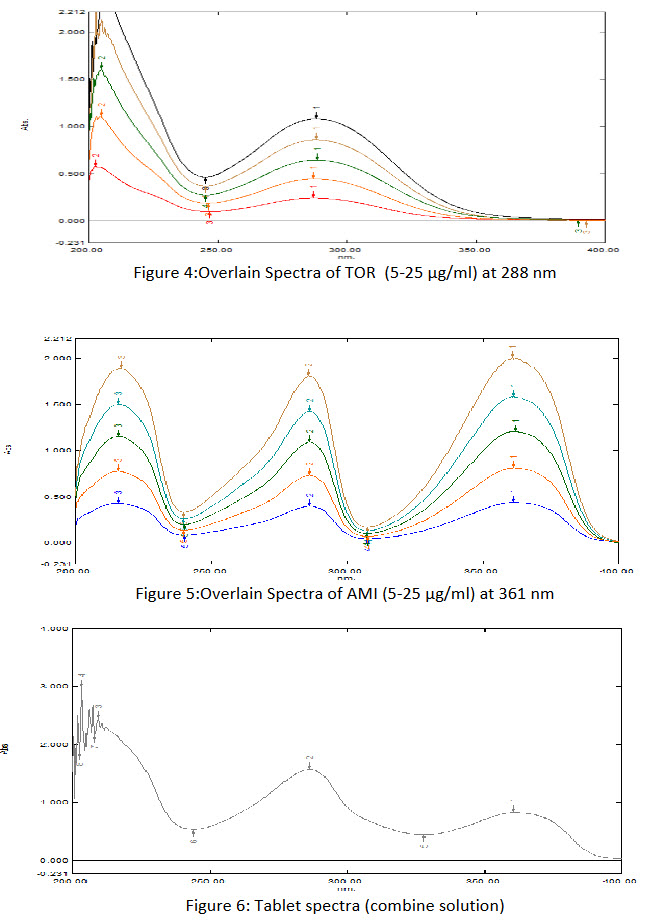
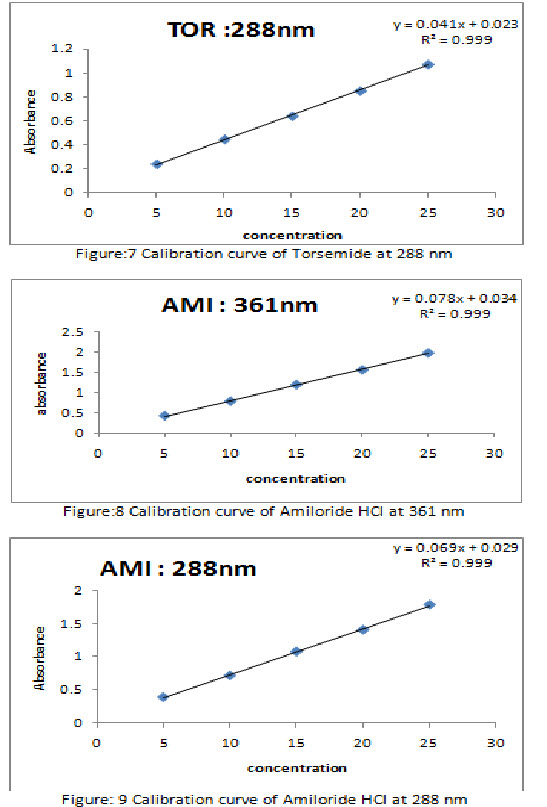
Table:1Linearity data for TOR and AMI
|
Drug |
Conc. (μg/ml) |
Absorbance mean ± S.D. (n = 5) |
%RSD |
||
|
|
288nm |
288nm |
|||
|
TORSEMIDE |
5 |
0.236 ±0.0023 |
0.99 |
||
|
10 |
0.444 ±0.000471 |
0.10 |
|||
|
15 |
0.693 ±0.0063 |
0.99 |
|||
|
20 |
0.852 ±0.0053 |
0.62 |
|||
|
25 |
1.075 ±0.0065 |
0.61 |
|||
|
|
288nm |
361nm |
288nm |
361nm |
|
|
AMILORIDE HCl |
5 |
0.391±0.000816 |
0.438 ±0.000471 |
0.20 |
0.10 |
|
10 |
0.717 ±0.00524 |
0.808 ±0.0063 |
0.73 |
0.76 |
|
|
15 |
1.080 ±0.004989 |
1.214 ±0.0069 |
0.46 |
0.57 |
|
|
20 |
1.411 ±0.005354 |
1.585 ±0.0037 |
0.37 |
0.23 |
|
|
25 |
1.791 ±0.0066
|
2.005 ±0.0016 |
0.36 |
0.08 |
|
Table: 2 Regression data for TOR and AMI
|
Regression Parameters |
TOR |
AMI |
|
|
288 nm |
288nm |
361nm |
|
|
Correlation coefficient (R2) |
0.9995 |
0.9994 |
0.9995 |
|
Slope (m) |
0.0417 |
0.0699 |
0.0783 |
|
Intercept (c) |
0.0234 |
0.0298 |
0.0342 |
Table:3 Data indicating recovery studies of Torsemide and Amiloride HCl
|
%Level of recovery |
Amount of drug taken (μg/ml) |
Amount of drug added (μg/ml) |
Total amount found (μg/ml) ± S.D. (n=3) |
% Recovery |
||||
|
|
TOR |
AMI |
TOR |
AMI |
TOR |
AMI |
TOR |
AMI |
|
80% |
10 |
5 |
8 |
4 |
17.91 |
9.1 |
99.53 |
101.11 |
|
100% |
10 |
5 |
10 |
5 |
19.69 |
10.02 |
98.48 |
100.20 |
|
120% |
10 |
5 |
12 |
6 |
22.2 |
11.12 |
101.08 |
101.09 |
Table: 4 Analysis of marketed formulation
|
Tablet |
Drug |
Label claim (mg) |
Amount found (mg) |
% label claim |
|
Toesinex-A |
Torsemide |
10 |
9.89 |
98.90% |
|
Amiloride HCl |
5 |
5.015 |
100.3% |
REFERENCE
1.Complete Torsemide drug information from drugs.com,2013 drugs.com/ppa/torsemide.html
2.O’Neil M. J., Smith A., Heckelman P. E. and Kinneary J. F. The Merk Index, an Encyclopedia of Chemicals, Drugs and Biologicals. Merck & Co. Inc., White House Station, New Jersey. 1996; 4th edition, 9552 .
3.Drugbank, “Open data Drug and Drug target Database” jun-2005, drugbank.ca/drugs/DB00594
4.O’Neil M. J., Smith A., Heckelman P. E. and Kinneary J. F. The Merk Index, an Encyclopedia of Chemicals, Drugs and Biologicals. Merck & Co. Inc., White House Station, New Jersey. 1996; 4th edition,406.
5.USP NF 2004, Asian Edition, authority of United States Pharmacopoeial convention, published by the board of trustees, 1869.
6.Indian pharmacopoeia-2010, addendum-2012, Government of India Ministry of Health and Family Welfare, published by Indian Pharmacopoeia commission, 2012: Vol-1, 2875-2876.
7.USP NF 2004, Asian Edition, authority of United States Pharmacopoeial convention, published by the board of trustees, 112.
8.ICH, Q2B. Validation of Analytical Procedure: Methodology. International Conference on Harmonization, IFPMA,Geneva. 2005
9.Medical news today, “hypertension”, 2013, medicalnewstoday.com/articles/150109.php
10.Complete Torsemide drug information from drugs.com,2013
drugs.com/ppa/torsemide.html
11.Cleveland clinic,“Heart Disease: Your Guide to Heart Failure”, October 2013, medicinenet.com/heart_failure/article.htm
12.Navas JP, Martinez-Maldonado M., “Pathophysiology of edema in congestive heart failure”,NationalCenter for Biotechnology Information;1993;2(4):325-329
REFERENCE ID: PHARMATUTOR-ART-2184
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 6 Received On: 10/04/2014; Accepted On: 18/04/2014; Published On: 01/06/2014 How to cite this article: DG Maheshwari, RD Patel; Absorption Correction Spectrophotometric Method for Simultaneous Estimation of Torsemide and Amiloride Hydrochloride in Their Combined Dosage Form; PharmaTutor; 2014; 2(6); 123-131 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









