ANAPHYLACTIC SHOCK: SHOCKING ERROR OF IMMUNE SYSTEM!
About Author:
Arvind Negi
Centre for Chemical and Pharmaceutical Sciences
Central University of Punjab, Bathinda-151001
arvindnegi2301@gmail.com
{ DOWNLOAD AS PDF }
Abstract
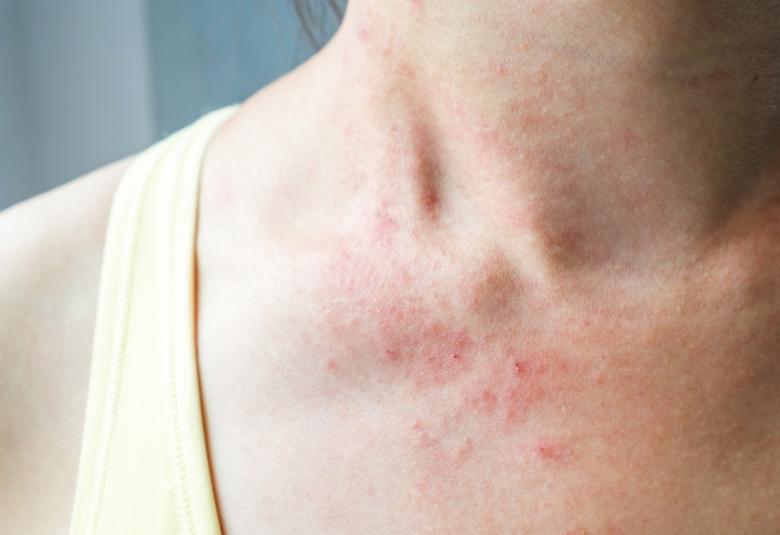
Activation of immune response is developed in respect to some xenobiotic sensitization, which is supposed to prevent and protect the body from sufferings induced by these xenobiotic. But unfortunately sometime body behaves in a paradoxical manner which misguides the immune system and ultimately turned into a bizarre situation of immune function. One of such response is anaphylactic shock, which is quite fatal if untreated. This review brings insights into the molecular physiology of anaphylactic shock and how some people elicit allergic response to certain substances but not the other. This major catastrophe of the body holds an error of immune system whereas this commentary based review disclosed the synchronization of the unfortunate lessons of the immune system, which makes it a cruel to cause DEATH!


 ABOUT AUTHORS:
ABOUT AUTHORS: 
 About Authors:
About Authors: 
 ABOUT AUTHORS:
ABOUT AUTHORS:





