
The Covid-19 pandemic has brought the spotlight on the health-promotive and disease-preventive solutions of Ayush disciplines. What has not come into the limelight is the emerging nation-wide trend in the Ayush disciplines, of taking up evidence-based studies.

CDSCO (Central Drugs Standard Control Organization) has developed draft guidelines for vaccine development in light of the present pandemic situation in the country. Development of vaccine is a complex activity which involves multidisciplinary research and generation of adequate laboratory, nonclinical and clinical data to ensure their safety, efficacy and quality. India is one of the major producers of vaccines in the world which cater the major portion of requirements of many vaccines globally.

Central Drugs Standard Control Organisation has approved phase II trials of Bharat Biotech's vaccine candidate Covaxin. After satisfactory results of Phase I clinical trial, DCGI has given permission for phase II trials of Covaxin, which is developed by Bharat biotech in alliance with the ICMR - National Institute of Virology.
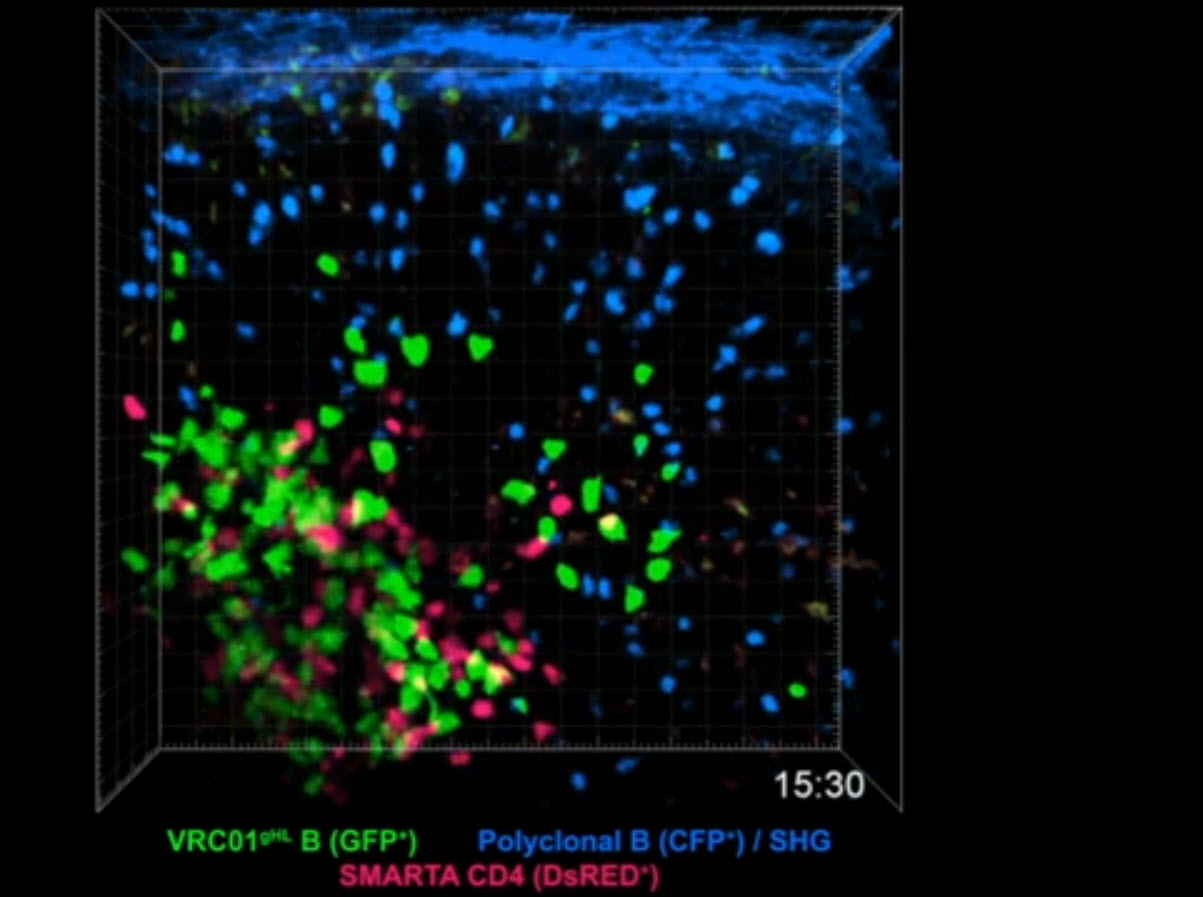
Designing a vaccine starts with finding the right ingredients. Every infectious agent has molecules, called antigens, that the immune system could potentially recognize and attack. So scientists must carefully consider which antigens should go into a vaccine.