Zydus receives Emergency Use Approval from DCGI for the use of Pegylated Interferon alpha-2b, Virafin
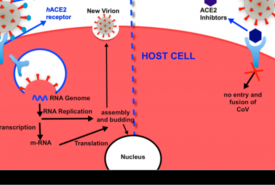
Zydus Cadila announced that the company has received Restricted Emergency Use Approval from the Drug Controller General of India (DCGI) for the use of Virafin, Pegylated Interferon alpha-2b (PegIFN) in treating moderate COVID-19 infection in adults. A single dose subcutaneous regimen of the antiviral Virafin will make the treatment more convenient for the patients. When administered early on during COVID, Virafin will help patients recover faster and avoid much of the complications.