IISc start-up gets regulatory approvals for COVID-19 test
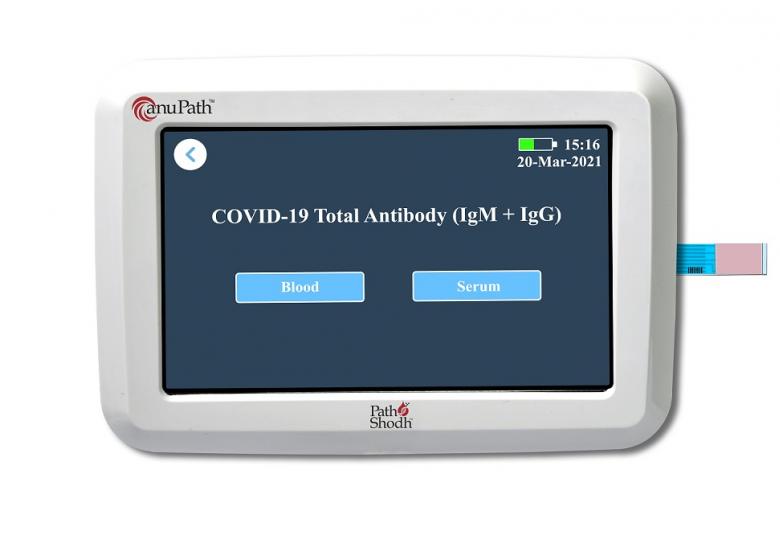
PathShodh Healthcare, a start-up incubated at the Society for Innovation and Development (SID), Indian Institute of Science (IISc), has made a significant breakthrough in developing a first-of-its-kind, semi-quantitative electrochemical ELISA test for COVID-19 IgM and IgG antibodies.