OKYO Pharma Limited announces First-in-Class Drug Candidate OK-101 Displaying Both Anti-inflammatory and Ocular Pain-Reducing Potential to Treat Dry Eye Disease
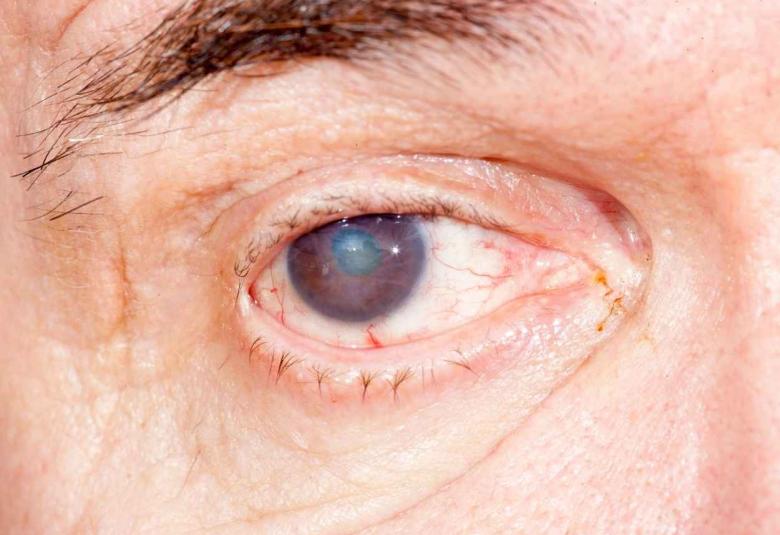
OKYO Pharma Limited a biotechnology company focused on the discovery and development of novel molecules to treat inflammatory dry eye diseases (DED) and ocular pain, is pleased to announce that its drug candidate OK-101 which was developed to treat DED through its anti-inflammatory mode of action also shows potent ocular pain reducing property determined using a mouse model of corneal neuropathic pain, establishing the potential to treat both pain and inflammation, the most common symptoms of dry eye, with a single drug.