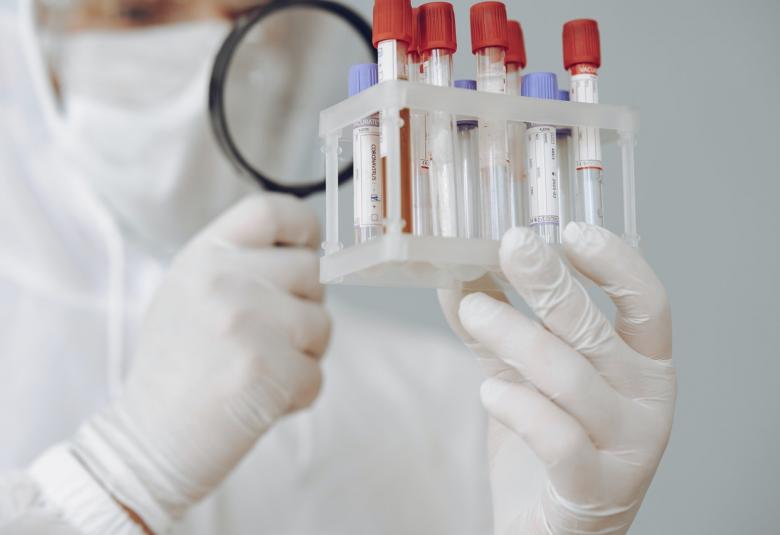
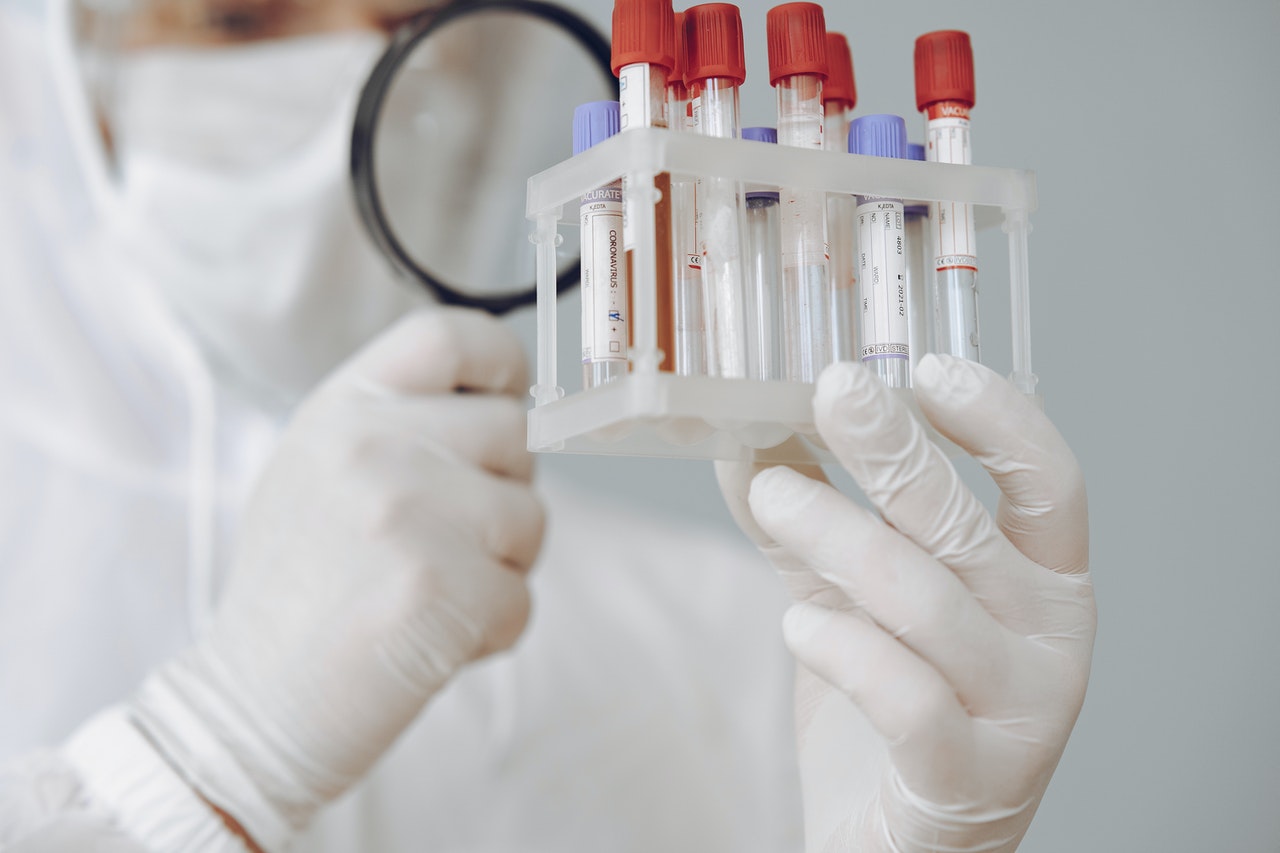
BASIC INFORMATION ABOUT RAPID TEST KITS, PCR TEST & POOL TESTING FOR CORONA
Dr. Tarun Virmani
Associate Professor
School of Pharmaceutical Sciences
MVN University, Palwal( Haryana)
Test kits identifies antibodies that are formed after the virus reaches the body & examining the antibody indicates whether the corona virus was reached in a person's body. Two methods are also used for this. Some kits identify IgM type antibodies (IgM is the first class of immunoglobulin made by B cells as they mature, and it is the form most commonly present as the antigen receptor on the B-cell surface. When IgM is secreted from the cells, five of the basic Y-shaped units become joined together to make a large pentamer molecule with 10 antigen-binding sites.