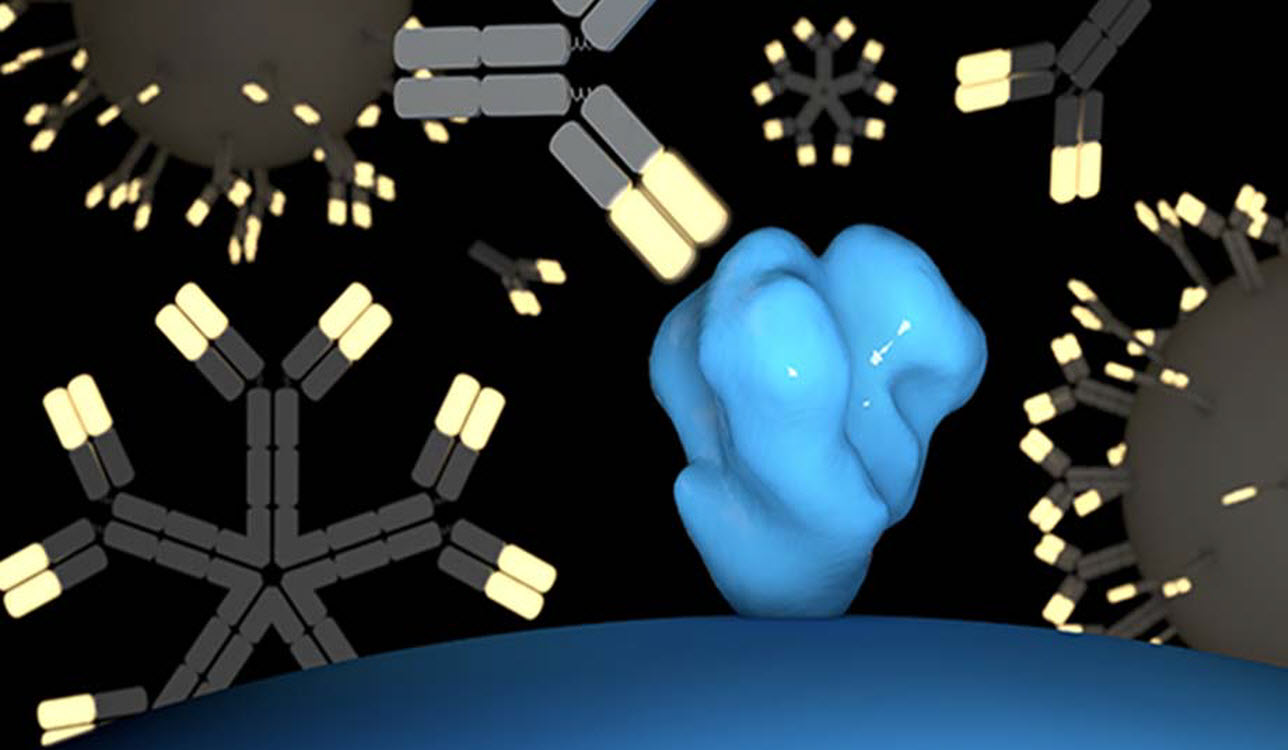
Antibodies from engineered B cells approach an HIV particle (shown in blue) to disable it. In a new study, scientists found that gene-edited B cells can generate durable protective antibody responses against HIV when activated with a vaccine. (Illustration courtesy of the Voss laboratory at Scripps Research.)