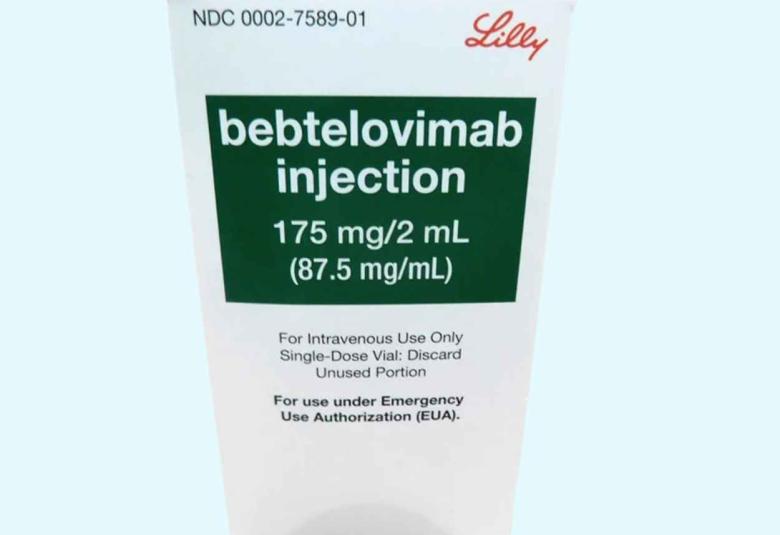
FDA lays aside Lillys Bebtelovimab in the U.S.
FDA lays aside Lilly's bebtelovimab in the U.S. for emergency use treatment of mild-to-moderate COVID-19 in adults and pediatric patients. Over the last several months, prevalence of COVID variant sublineages vary by state, region and even country, and can change rapidly.
Lilly said that it agrees with the FDA that it is not medically appropriate, at this time, to treat high-risk patients with mild-to-moderate COVID-19 with bebtelovimab in the US.