Discovery could lead to new drugs to block protein that fuels bowel cancer
Scientists have revealed the inner workings of a key protein involved in a wide range of cellular processes – potentially paving the way for better and less toxic cancer drugs.
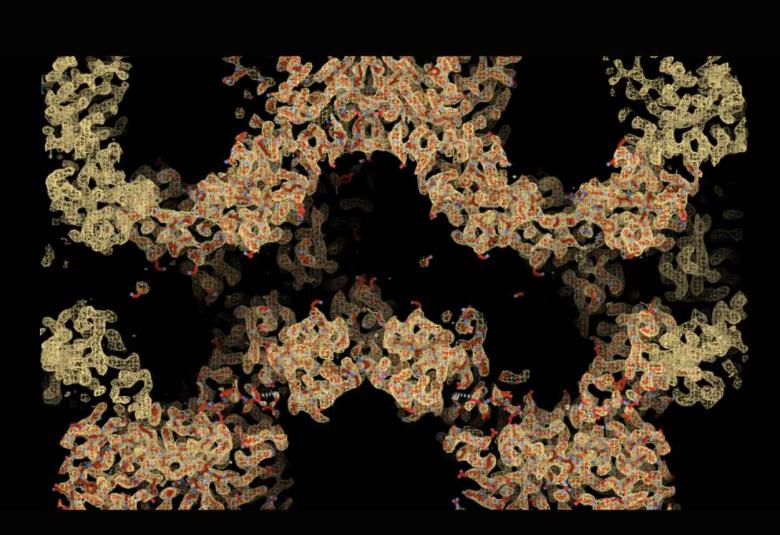
Using Nobel Prize-winning microscopy techniques, the researchers revealed how the tankyrase protein switches itself on and off by self-assembling into 3D chain-like structures.