Q.2. (b) Show how you would distinguish between the following by spectroscopy
(i) Aldehyde and Ketone
(ii) Alcohol and Ether
(iii) Mono substituted benzene and Di-substituted benzene
[adsense:336x280:8701650588]
(i). Aldehyde and Ketone
Highly polar C=O bond (1710 - 1720 cm-1) (present in both aldehydes & ketones)
C-H bond sp2 carbon (2700-2800 cm-1) (present only in aldehydes)
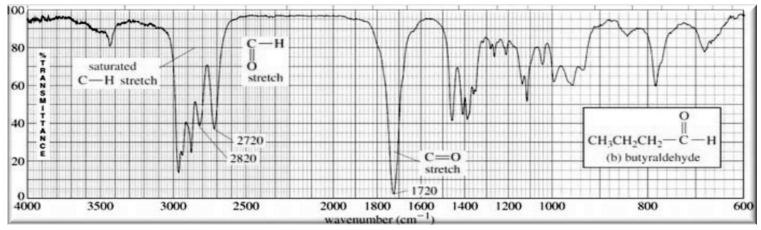
Figure 2.3 (butyraldehyde)

Figure 2.4 (2-heptanone)
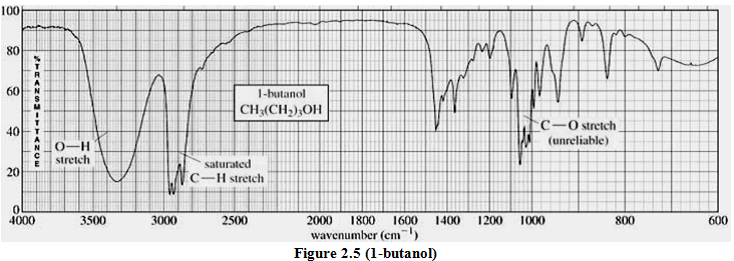
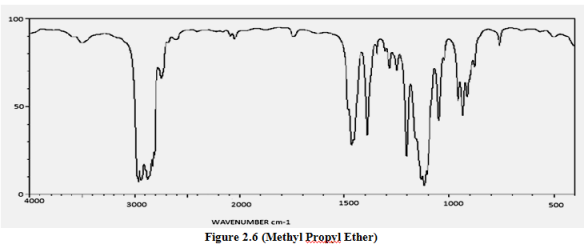
(ii). Alcohol & Ether
IR spectra:
C—O bond 1050-1275 cm-1
1o ROH 1050
2o ROH 1100
3o ROH 1150
ethers 1060-1150
O—H bond 3200-3640 cm-1
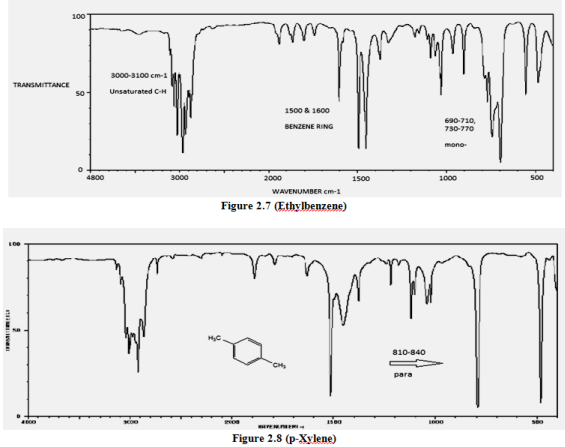
(iii). Mono & Di-substituted benzene
IR spectra:
=C—H bond, “unsaturated” “aryl”
(sp2) 3000-3100 cm-1
+ 690-840
Mono-substituted + 690-710, 730-770
ortho-disubstituted + 735-770
meta-disubstituted + 690-710, 750-810(m)
para-disubstituted + 810-840(m)
C=C bond 1500, 1600 cm-1