(a) Explain ATR phenomenon and its applications
Ans.2.(a) ATRInternal reflection spectroscopy is a technique for obtaining IR spectra of samples that are difficult to deal with, such as solids of limited solubility, films, threads, pastes, adhesives, and powders. Principle when a beam of radiation passes from a denser to a less dense medium, reflection occurs. The fraction of the incident beam reflected increases as the angle of incidence becomes larger; beyond a certain critical angle, reflection is complete. It has been shown both theoretically and experimentally that during the reflection process the beam penetrate a small distance into the less dense medium before reflection occurs.
[adsense:336x280:8701650588]
The depth of penetration, which varies from a fraction of a wavelength up to several wavelengths, depends on the wavelengths, the index of reflection of the two materials, and angle of the beam with respect to the interface. The penetration radiation is called the evanescent wave at wavelengths where the less dense medium absorbs the evanescent radiation, attenuation of the beam occurs, which is known as attenuated total reflectance (ATR). The resulting ATR spectrum resembles that of a conventional IR spectrum with some differences.
Instrumentation:
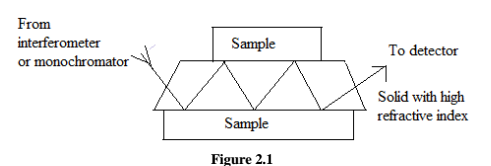
An apparatus for ATR measurements shown fig 2.1,as can be seen from the upper figure, the sample (solid) is placed on opposite sides of a transparent crystalline material of high refractive index. By proper adjustment of the incident angle, the radiation undergoes multiple internal reflections before passing from the crystal to detector. Absorption and attenuation take place at each of these reflections.

Figure 2.2 is an optical diagram of an adapter that fits into the cell area of most IR spectrometers and permits ATR measurements. Cells for liquid samples are also available.
ATR spectra: ATR spectra are similar but not identical to ordinary absorption spectra. In general, although the same bands are observed, their relative intensities differ. With ATR spectra, the absorbance, although dependent on the angle of incidence, are independent of the sample thickness, because the radiation penetrates only a few micrometers into the sample. The effective penetration depth dpdepends on the wavelength of the beam, the refractive indexes of the crystal and the sample, the beam angle,the penetration depth can be calculated from dp = λc / 2π [sin2θ - (ns/nc)2]1/2 Where λc is the wavelength in the crystal (λ/nc),θ is the angle of incidence, and ns and nc are the refractive indexes of the sample and crystal, respectively.
Application of ATR:
1. Major advantage of ATR spectroscopy is that absorption spectra are readily obtainable on a wide variety of sample types with a minimum of preparation.
2. Threads, yarns, fabrics and fibers can be studied by pressing the samples against the dense crystal.
3. Pastes, powders, or suspensions can be handled in a similar way.
4. Aqueous solutions can also be accommodated provided the crystal is not water soluble. There are even ATR flow cell available.
5.ATR spectroscopy has been applied to many substances, such as polymers, rubbers, and other solids.









