 About Authors:
About Authors:
Patel Chirag J*1, Prof. Satyanand Tyagi2, Patel Pinkesh1, Umesh Kumar1, Patel Jaimin1, Chaudhari Bharat1
1Department of Pharmaceutics, Maharishi Arvind Institute of Pharmacy, Mansarovar, Jaipur, Rajasthan, India-302020.
*Mr. Chirag Patel has published various Books, Research and Review articles. His academic works include 35 Publications (2 books was published in Lambert Academic Publishing, Germany & 8 Research Articles and 25 Review Articles was published in standard and reputed National and International Pharmacy journals)
2President & Founder, Tyagi Pharmacy Association (TPA) & Scientific Writer (Pharmacy), Chattarpur, New Delhi, India-110074.
*chirag.bangalore@gmail.com, +91-8000501871
ABSTRACT:
The transdermal route of drug delivery has gained great interest of pharmaceutical research, as it circumvents number of problems associated with oral route of drug administration. Recently, various strategies have been used to augment the transdermal delivery of bioactives. Mainly, they include electrophoresis, iontophoresis, chemical permeation enhancers, microneedles, sonophoresis, and vesicular system like liposomes, niosomes, elastic liposomes such as ethosomes and transfersomes. Among these strategies transferosomes appear promising. A novel vesicular drug carrier system called transfersomes, which is composed of phospholipid, surfactant, and water for enhanced transdermal delivery. Transfersomes are a form of elastic or deformable vesicle, which were first introduced in the early 1990s.The system can be characterized by in vitro for vesicle shape and size, entrapment efficiency, degree of deformability, number of vesicles per cubic mm. These carriers can transport pharmacological agents, including large polypeptides, through the permeability barriers, such as the intact skin.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1572
INTRODUCTION
Delivery via the transdermal route is an interesting option in this respect because a transdermal route is convenient and safe. Transdermal route offers several potential advantages over conventional routes like avoidance of first pass metabolism, predictable and extended duration of activity, minimizing undesirable side effects, utility of short half-life drugs, improving physiological and pharmacological response, avoiding the fluctuation in drug levels, inter-and intra-patient variations, and most importantly, it provides patients convenience [1].
Vesicles have a unique structure which is capable of entrapping hydrophilic, lipophilic, amphiphilic and charged hydrophilic drugs. Vesicles are colloidal particles having a water filled core surrounded by a wall of lipids and surfactants (amphiphiles) arranges in bilayer. If the proportion of water is increased, these amphiphiles can form one or more concentric bilayers. Hydrophilic drugs find a place in the internal aqueous environment while amphiphilic, lipophilic drugs get entrapped in the bilayered wall with electrostatic and/or hydrophobic forces. The flexible or deformable vesicles are called elastic vesicles or Transfersomes [2].
Transfersomes are advantageous as phospholipids vesicles for transdermal drug delivery. Because of their self-optimized and ultra flexible membrane properties, they are able to deliver the drug reproducibly either into or through the skin, depending on the choice of administration or application, with high efficiency. The vesicular transfersomes are more elastic than the standard liposomes and thus well suited for the skin penetration. Transfersomes overcome the skin penetration difficulty by squeezing themselves along the intracellular sealing lipid of the stratum corneum. This mechanism can be well understood from the Fig. 1. These are characteristic with transfersomes, because of the high vesicle deformability, which permits the entry due to the mechanical stress of surrounding, in a self-adapting manner. Flexibility of transfersomes membrane is governed by mixing suitable surface-active components in the proper ratios with phospholipids [3]. The resulting flexibility of transfersome membrane minimizes the risk of complete vesicle rupture in the skin and allows transfersomes to follow the natural water gradient across the epidermis, when applied under non-occlusive condition. Transfersomes (Fig. 2) can penetrate the intact stratum corneum spontaneously along two routes in the intracellular lipid that differ in their bilayers properties [4]. Bangham discovered liposomes in 1963 and since then vesicular systems have attracted increasing attention [5]. But recently it has become evident that classic liposomes are of minor values in terms of penetration. Confocal microscopic studies have shown that intact liposomes are not able to penetrate into granular layer of epidermis but, they rather remain on the upper layer of stratum corneum. The modification of the vesicular compositions or surface properties can adjust the drug release rate and the deposition to the target site [6].
[adsense:468x15:2204050025]
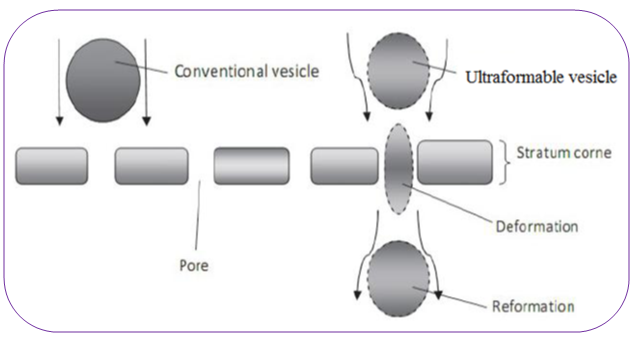
Figure 1: Deformability of transfersomes in to skin pores [2].

Figure 2: Structural representation of one transfersome unit [7].
SILENT FEATURES OF TRANSFERSOMES
Transfersomes can deform and pass through narrow constriction (from 5 to 10 times less than their own diameter) without measurable loss.
1. This high deformability gives better penetration of intact vesicles.
2. Transfersomes possess an infrastructure consisting of hydrophobic and hydrophilic moieties together and as a result can accommodate drug molecules with wide range of solubility.
3. They can act as a carrier for low as well as high molecular weight drugs e.g. analgesic, anesthetic, corticosteroids, sex hormone, anticancer, insulin, gap junction protein, and albumin.
4. They are biocompatible and biodegradable as they are made from natural phospholipids similar to liposomes.
5. They have high entrapment efficiency, in case of lipophilic drug near to 90%.
6. They protect the encapsulated drug from metabolic degradation.
7. They act as depot, releasing their contents slowly and gradually.
8. Easy to scale up, as procedure is simple, do not involve lengthy procedure and unnecessary use or pharmaceutically unacceptable additives.
9. They can be used for both systemic as well as topical delivery of drug [1, 7, 8].
LIMITATIONS OF TRANSFERSOMES
1. Transfersomes are chemicallyunstable because of theirpredisposition to oxidativedegradation.
2. Transfersomes formulations are expensive
3. Purity of natural phospholipids is another criteria militating against adoption of transfersomes as drug delivery vehicles [2, 7, 8].
PREPARATION OF TRANSFERSOMES
Various published and patented procedure are available for the preparation of transfersome. Generally phosphatidylcholine is mixed in ethanol with sodium cholate or some other biocompatible surfactant. Subsequently a suitable buffer is added to yield a total lipid concentration of 10% w/w. the suspension is then sonicated, frozen, and thawed 2-3 times to catalyze vesicle growth and is finally brought to the preferred vesicle size by pressure homogenization, ultrasonication, or some other mechanical method. Final vesicle size, as determined dynamic light scattering, is approximately 120 nm for a typical transfersome preparation containing 8.7% by weight SPC, 1.3% by weight sodium cholate, and up to 8.5% by volume ethanol. The best carrier composition has to be found experimentally and for each drug separately to obtain appropriate transfersome carriers with maximum deformability and stability [1, 3].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 1: Different additives used in formulation of transfersome [1, 8]
|
Class |
Example |
Uses |
|
Phospholipids |
Soya phosphatidyl choline, Dipalmitoyl phosphatidyl choline, Distearoyl phoshatidyl choline |
Vesicles forming component |
|
Surfactant |
Sod. Cholate, Sod.deoxycholate, tween-80, Span-80 |
For providing flexibility |
|
Alcohol |
Ethanol, methanol |
As a solvent |
|
Buffering agent |
Saline phosphate buffer (pH 6.4) |
As a hydrating medium |
|
Dye |
Rhodamine-123, Rhodamine-DHPE, Fluorescein-DHPE Nilered |
For CSLM study |
CHARACTERIZATION OF TRANSFERSOMES
The characterization of transfersomes is generally similar to liposomes, niosomes and micelles [9].Following characterization parameters have to be checked for transfersomes.
1. Entrapment efficiency
The entrapment efficiency is expressed as the percentage entrapment of the drug added. Entrapment efficiency was determined by first separation of the un-entrapped drug by use of mini-column centrifugation method. After centrifugation, the vesicles were disrupted using 0.1% Triton X-100 or 50% n-propanol [1]. The entrapment efficiency is expressed as:
Entrapment efficiency = (Amount entrapped / Total amount added) ×100
2. Drug content
The drug content can be determined using one of the instrumental analytical methods such as modified high performance liquid chromatography method (HPLC) method using a UV detector, column oven, auto sample, pump, and computerized analysis program depending upon the analytical method of the pharmacopoeial drug [10].
3. Vesicle size distribution and zeta potential
Vesicle size, size distribution and zeta potential were determined by Dynamic Light Scattering Method (DLS) using a computerized inspection system by Malvern Zetasizer [1, 2].
4. Vesicle morphology
Vesicle diameter can be determined using photon correlation spectroscopy or dynamic light scattering (DLS) method. Samples were prepared in distilled water, filtered through a 0.2 mm membrane filter and diluted with filtered saline and then size measurement done by using photon correlation spectroscopy or dynamic light scattering (DLS) measurements. Transfersomes vesicles can be visualized by TEM, phase contrast microscopy, etc. The stability of vesicle can be determined by assessing the size and structure of vesicles over time. Mean size is measured by DLS and structural changes are observed by TEM [1, 2].
5. No. of vesicles per cubic mm
This is an important parameter for optimizing the composition and other process variables. Non-sonicated transfersome formulations are diluted five times with 0.9% sodium chloride solution. Haemocytometer and optical microscope can then be used for further study [8]. The Transfersomes in 80 small squares are counted and calculated using the following formula:
Total number of Transfersomes per cubic mm = (Total number of Transfersomes counted × dilution factor × 4000) / Total number of squarescounted
6. Confocal scanning laser microscopy study
Conventional light microscopy and electron microscopy both face problem of fixation, sectioning and staining of the skin samples. Often the structures to be examined are actually incompatible with the corresponding processing techniques; these give rise to misinterpretation, but can be minimized by Confocal Scanning Laser Microscopy (CSLM). In this technique lipophilic fluorescence markers are incorporated into the transfersomes and the light emitted by these markers used for following purpose:
1. For investigating the mechanism of penetration of transfersomes across the skin,
2. For determining histological organization of the skin (epidermal columns, interdigitation), shapes and architecture of the skin penetration pathways.
3. For comparison and differentiation of the mechanism of penetration of transfersomes with liposomes, niosomes and micelles.
Different fluorescence markers used in CSLM study are as:
1. Fluorescein- DHPE (1, 2- dihexadecanoyl- sn- glycero- 3- phosphoethanolamine- N- (5-fluoresdenthiocarbamoyl), triethyl- ammonium salt)
2. Rhodamine- DHPE (1, 2- dihexadecanoyl- sn- glycero- 3ogisogietgabikanube-LissamineTmrhodamine-B- sulfonyl), triethanol- amine salt)
3. NBD- PE (1, 2- dihexadecanoyl- sn-glycero- 3- phosphoethanolamine- N- (7-nitro- Benz- 2- xa- 1,3- diazol- 4- yl) triethanolamine salt)
4. Nile red [4].
7. Turbidity measurement
Turbidity of drug in aqueous solution can be measured using nephelometer [1].
8. Degree of deformability or permeability measurement
In the case of transfersomes, the permeability study is one of the important and unique parameter for characterization. The deformability study is done against the pure water as standard. Transfersomes preparation is passed through a large number of pores of known size (through a sandwich of different microporous filters, with pore diameter between 50 nm and 400 nm, depending on the starting transfersomes suspension). Particle size and size distributions are noted after each pass by dynamic light scattering (DLS) measurements [1, 11].
9. Penetration ability
Penetration ability of Transfersomes can be evaluated using fluorescence microscopy [8, 11].
10. Occlusion effect
Occlusion of skin is considered to be helpful for permeation of drug in case of traditional topical preparations. But the same proves to be detrimental for elastic vesicles. Hydrotaxis (movement in the direction) of water is the major driving force for permeation of vesicles through the skin, from its relatively dry surface to water rich deeper regions. Occlusion affects hydration forces as it prevents evaporation of water from skin [1].
11. Surface charge and charge density
Surface charge and charge density of Transfersomes can be determined using zetasizer [1, 8].
12. Physical stability
The initial percentage of the drug entrapped in the formulation was determined and were stored in sealed glass ampoules. The ampoules were placed at 4 ± 20C (refrigeration), 25 ± 20C (room temp), and 37 ± 20C (body temp) for at least 3 months. Samples from each ampoule were analyzed after 30 days to determine drug leakage. Percent drug lose was calculated by keeping the initial entrapment of drug as 100% [10, 12].
13. In-vitro drug release
In vitro drug release study is performed for determining the permeation rate. Time needed to attain steady state permeation and the permeation flux at steady state and the information from in-vitro studies are used to optimize the formulation before more expensive in vivo studies are performed. For determining drug release, transfersomes suspension is incubated at 320C and samples are taken at different times and the free drug is separated by mini column centrifugation. The amount of drug released is then calculated indirectly from the amount of drug entrapped at zero times as the initial amount (100% entrapped and 0% released) [1, 8].
14. In-vitro Skin permeation Studies
Modified Franz diffusion cell with a receiver compartment volume of 50ml and effective diffusion area of 2.50 cm2 was used for this study. In vitro drug study was performed by using goat skin in phosphate buffer solution (pH 7.4). Fresh Abdominal skin of goat were collected from slaughterhouse and used in the permeation experiments. Abdominal skin hairs were removed and the skin was hydrated in normal saline solution. The adipose tissue layer of the skin was removed by rubbing with a cotton swab. Skin was kept in isopropyl alcohol solution and stored at 0-40?C.
To perform skin permeation study, treated skin was mounted horizontally on the receptor compartment with the stratum corneum side facing upwards towards the donor compartment of Franz diffusion cell. The effective permeation area of donor compartment exposed to receptor compartment was 2.50cm2 and capacity of receptor compartment was 50ml. The receptor compartment was filled with 50ml of phosphate buffer (pH 7.4) saline maintained at 37 ± 0.5?C and stirred by a magnetic bar at 100RPM. Formulation (equivalent to 10mg drug) was placed on the skin and the top of the diffusion cell was covered. At appropriate time intervals 1 ml aliquots of the receptor medium were withdrawn and immediately replaced by an equal volume of fresh phosphate buffers (pH 7.4) to maintain sink conditions. Correction factors for each aliquot were considered in calculation of release profile. The samples were analyzed by any instrumental analytical technique [13, 14].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
APPLICATION OF TRANSFERSOMES
1. Delivery of proteins and peptides:
Transfersomes have been widely used as a carrier for the transport of proteins and peptides. Proteins and peptide are large biogenic molecules which are very difficult to transport into the body, when given orally they are completely degraded in the GI tract. These are the reasons why these peptides and proteins still have to be introduced into the body through injections. Various approaches have been developed to improve these situations. The bioavaibility obtained from transferosomes is somewhat similar to that resulting from subcutaneous injection of the same protein suspension. The transferosomal preparations of this protein also induced strong immune response after the repeated epicutaneous application, for example the adjuvant immunogenic serum albumin in transferosomes, after several dermal challenges is as active immunologically as is the corresponding injected proteo-transferosomes preparations [15, 16].
2. Delivery of insulin:
By transferosomes is the successful means of non invasive therapeutic use of such large molecular weight drugs on the skin. Insulin is generally administered by subcutaneous route that is inconvenient. Encapsulation of insulin into transferosomes (transfersulin) overcomes these entire problems. After transfersulin application on the intact skin, the first sign of systemic hypoglycemia are observed after 90 to 180 min, depending on the specific carrier composition [17].
3. Delivery of corticosteroids: Transferosomes have also used for the delivery of corticosteroids. Transferosomes improves the site specificity and overall drug safety of corticosteroid delivery into skin by optimizing the epicutaneously administered drug dose. Transferosomes based corticosteroids are biologically active at dose several times lower than the currently used formulation for the treatment of skin diseases [3].
4. Delivery of interferons:
Transferosomes have also been used as a carrier for interferons, for example leukocytic derived interferone-α (INF-α) is a naturally occurring protein having antiviral, antiproliferive and some immunomodulatory effects. Transferosomes as drug delivery systems have the potential for providing controlled release of the administered drug and increasing the stability of labile drugs. Hafer et al studied the formulation of interleukin-2 and interferone-α containing transferosmes for potential transdermal application .they reported delivery of IL-2 and INF- α trapped by transferosomes in sufficient concentration for immunotherapy [18].
5. Delivery of anesthetics:
Application of anesthetics in the suspension of highly deformable vesicles, transferosomes, induces a topical anesthesia, under appropriate conditions, with less than 10 min. Maximum resulting pain insensitivity is nearly as strong (80%) as that of a comparable subcutaneous bolus injection, but the effect of transferosomal anesthetics last longer [2].
6. Transdermal immunization:
Another most important application of transferosomes is transdermal immunization using transferosomes loaded with soluble protein like integral membrane protein, human serum albumin, and gap junction protein. These approaches offer at least two advantages, first they are applicable without injection and second, they give rise to rather high titer and possibly, to relatively high IgA levels [2].
7. Delivery of NSAIDS:
NSAIDS are associated with number of GI side effects. These can be overcome by transdermal delivery using ultra-deformable vesicles. Studies have been carried out on Diclofenac and Ketoprofen. Ketoprofen in a Transfersome formulation gained marketing approval by the Swiss regulatory agency (SwissMedic) in 2007; the product is expected to be marketed under the trademark Diractin. Further therapeutic products based on the Transfersome technology, according to IDEA AG, are in clinical development [19].
8. Delivery of Herbal Drugs:
Transfersomes can penetrate stratum corneum and supply the nutrients locally to maintain its functions resulting maintenance of skin in this connection the Transfersomes of Capsaicin has been prepared by Xiao-Ying et al. which shows the better topical absorption in comparison to pure capsaicin [20].
9. Delivery of Anticancer Drugs:
Anti cancer drugs like methotrexate were tried for transdermal delivery using transfersome technology. The results were favorable. This provided a new approach for treatment especially of skin cancer [2, 20].
DISCUSSION AND CONCLUSION
Transdermal drug delivery is proving to be superior to the conventional oral delivery of drugs owing to its distinct virtues. Transfersomes are specially optimized particles or vesicles, which can respond to an external stress by rapid and energetically inexpensive, shape transformations. Such highly deformable particles can thus be used to bring drugs across the biological permeability barriers, such as skin. Transfersomes do not depend upon the concentration gradient and mainly works on the principle of hydrotaxis and elasto-mechanics. They can act as a carrier for low as well as high molecular weight drugs e.g. analgesic, anesthetic, corticosteroids, sex hormone, anticancer, insulin, gap junction protein, and albumin.
REFERENCES
1. Prajapati ST, Patel CG, Patel CN, “Transfersomes: A Vesicular Carrier System For Transdermal Drug Delivery”, Asian Journal of Biochemical and Pharmaceutical Research, 2011, 2 (1), 507-524.
2. Modi CD, Bharadia PD, “Transfersomes: New Dominants for Transdermal Drug Delivery”, Am. J. PharmTech Res., 2012, 2 (3), 71-91.
3. Cevc G, “Isothermal lipid phase”, Transitions Chemistry and Physics of Lipids, 1991, 57, 293-299.
4. Schatzlein A, Cevc G, “Skin penetration by phospholipids vesicles, Transfersomes as visualized by means of the Confocal Scanning Laser Microscopy, in characterization, metabolism, and novel biological applications”, AOCS Press, 1995, 191-209.
5. Bangham AD, “Physical structure and behavior of lipids and lipid enzymes”, Adv. Lipid Res., 1963, 1, 65-104.
6. Swarnlata S, Gunjan J, Chanchal DK, Shailendra S, “Development of novel herbal cosmetic cream with Curcuma longa extract loaded transfersomes for anti-wrinkle effect”, African J Pharm Pharmacol, 2011, 5 (8), 1054-1062.
7. Kombath RV, Minumula SK, Sockalingam A, Subadhra S, Parre S, Reddy TR, David B, “Critical issues related to transfersomes – novel Vesicular system”, Acta Sci. Pol., Technol. Aliment., 2012, 11 (1), 67-82.
8. Walve JR, Bakliwal SR, Rane BR, Pawar SP, “Transfersomes: A surrogated carrier for transdermal drug delivery system”, International Journal of Applied Biology and Pharmaceutical Technology, 2011, 2 (1), 201-214.
9. Panchagnula R, “Transdermal delivery of drugs”, Indian Journal Pharmacology, 1997, 29, 140-156.
10. Sheo DM, Shweta A, Ram CD, Ghanshyam M, Girish K, Sunil KP “Transfersomes- a Novel Vesicular Carrier for Enhanced Transdermal Delivery of Stavudine: Development, Characterization and Performance Evaluation”, J. Scientific Speculations and Res., 2010, 1 (1), 30-36.
11. Pandey S, Goyani M, Devmurari V, Fakir J, “Transferosomes: A Novel Approach for Transdermal Drug Delivery”, Der Pharmacia Lettre, 2009, 1 (2), 143-150.
12. Sheo DM, Shweta A, Vijay KT, Ram CD, Aklavya S, Ghanshyam M, “Enhanced Transdermal delivery of indinavir sulfate via transfersomes”, Pharmacie Globale (IJCP), 2010, 1 (06), 1-7.
13. Patel R, Singh SK, Singh S, Sheth NR, Gendle R, “Development and Characterization of Curcumin Loaded Transfersome for Transdermal Delivery” J. Pharm Sci. Res., 2009, 1 (4), 71-80.
14. Jalon GE. Ygartua P, Santoyo S, “Topical application of acyclovir-loaded microparticles: quantification of the drug in porcine skin layers”, J. Control Release, 2001, 75, 191-197.
15. Trotta M, Peira E, Carlotti ME, Gallarate M, “Deformable liposomes for dermal administration of methotrexate”, Int. J. Pharma, 2004, 270, 119.
16. Maghraby EI, Williams GM, Barry BW, “Skin delivery of oestradiol from lipid vesicles: importance of liposome structure”, Int. J. Pharma, 2000, 204 (1-2), 159-69.
17. Gregor C, Dieter G, Juliane S, Andreas S, Gabriele B, “Ultra-flexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin”, Biophysica Acta, 1998, 1368, 201-215.
18. Hafer C, Goble R, Deering P, Lehmer A, Breut J, “Formulation of interleukin-2 and interferon-alpha containing ultra-deformable carriers for potential transdermal application”, Anticancer Res., 1999, 19 (2c), 1505-7.
19. Dubey V, Mishra D, Asthana A, Jain NK, “Transdermal delivery of a pineal hormone: melatonin via elastic liposomes”, Biomaterials, 2006, 27 (18), 3491-6.
20. Benson HA, “Transfersomes for transdermal drug delivery”, Expert Opin. Drug Deliv., 2006, 3 (6), 727-37.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









