 ABOUT AUTHOR
ABOUT AUTHOR
Dr. R. S. Thakur
Chief Editor, Journal of Pharmaceutical Research
Krupanidhi College of Pharmacy, Bengaluru, India.
Email : drramsthakur@gmail.com
Preamble
On 5 th April, 1940 The Drugs Bill was passed by the Legislative Assembly of India and on 10th April, 1940 the Drugs Act, 1940 entered the Statute book of British India. This met a long overdue demand of immediate measures to control the craze for medicinal drugs by legislation for the standardisation of the preparation and sale of such drugs. It was rechristened Drugs and Cosmetics Act, 1940 w.e.f. 27-7-1964 by The Drugs (Amendment) Act, 1962 (XXI of 1962) by inserting the words “and Cosmetics” in the long title and first paragraph of the preamble after the word “Drugs”. This amendment also inserted the words “and Cosmetics” in sub-section (1) of section 1 of the principal Act after the word “Drugs”. It inserted definition of cosmetics as clause (aa) in section 3 of the principal Act, made corresponding amendments in sections 3, 6, 8, 10, 11, 12, 14, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 28, 29, 30, 31, and 33 relating to cosmetics and inserted new sections 9A, 17A and 27A in the principal Act. This Act has been amended time and again for expanding its scope and ensuring more rigid measures and control to fulfil its objectives of regulating the import, manufacture, distribution and sale of drugs and cosmetics. A chronological depiction of the amendments is as under:-
1. The Part B States (Laws) Act, 1951 (Act No. 3 of 1951), w.e.f. 1-4-1951.
2. The Drugs (Amendment) Act, 1955 (11 of 1955), w.e.f. 15-4-1955.
3. The Drugs (Amendment) Act, 1960 (35 of 1960), w.e.f. 16-3-1961.
4. The Drugs (Amendment) Act, 1962 (21 of 1962), w.e.f. 27-7-1964.
5. The Drugs and Cosmetics (Amendment) Act, 1964 (13 of 1964), w.e.f. 15-9-1964.
6. The Drugs and Cosmetics (Amendment) Act, 1972 (19 of 1972), w.e.f. 31-5-1972.
7. The Drugs and Cosmetics (Amendment) Act, 1982 (68 of 1982), w.e.f. 1-2-1983.
8. The Drugs and Cosmetics (Amendment) Act, 1986 (71 of 1986), w.e.f. 15-9-1987.
9. The Finance Act, 1995 (22 of 1995), w.e.f. 26-5-1995.
10. The Drugs and Cosmetics (Amendment) Act, 2008 (26 of 2008), w.e.f. 10-8-2009.
Reference Id: PHARMATUTOR-ART-3002
Historical background
The origin of demand to control standards of medicinal drugs can be traced in the Presidential address of Acharya Jagadish Chandra Bose delivered at 14th Indian Science Congress held in Lahore in January 1927. Taking cue from the deliberations at that session of Indian Science Congress, on 9th March 1927 The Honourable Sir Haroon Jaffer, member of Council of States [1] moved the following resolution:-

Speaking on the resolution of Sir Haroon Jafar, The Honourable Sir Maneckji Dadabhoy moved amendment to the original resolution. Concluding portion of his speech and the resolution moved is appended below:

As a consequence of the adoption of the amended resolution of The Honourable Sir Maneckji Dadabhoy; Government of India, Department of Education, Health and Lands, addressed letter No. 624-Health, dated the 19th April 1927 to All Local Governments on the issue of measures to control the preparation and sale of medicinal drugs.
The Local Governments of Madras, Bihar and Orissa, Burma, Assam, Central Provinces, United Provinces and Punjab communicated their views to the Department of Education, Health and Lands of Government of India vide their letters as under:-

Quality of Quinine Mixture
The May 1928 issue of The Indian Medical Gazette [2] published a paper on Stock Solutions of Quinine authored by M/s. Lt. Col. J. W. D. Megaw, Sudhamoy Ghosh and N. R. Chatterjee. The findings of the research paper were as follows:-

The Indian and Eastern Druggist, the Pharmaceutical Journal of England, reputed Newspaper The Statesman and the Civil and Military Gazette also came forward to support the need for legislation.
Adjournment Motion in Legislative Assembly
In spite of the sequence of events explained in the foregoing paragraphs, the then British regime failed to demonstrate any sign of legislative measure. In view of the dilly dallying, during the third Session of the third Legislative Assembly held at Shimla, on Tuesday, the 4th September, 1928 an Adjournment Motion [3] was moved by the Honourable Lt. Col. Henry A. J. Gidney on the issue of Quinine Fraud. The President of the Legislative Assembly (the Honourable Mr. Vithalbhai Javerbhai Patel) was in the Chair. In his introductory speech the Honourable Lt. Col. H. A. J. Gidney inter alia informed the House- “...
Most of the quinine offered in the bazaars for sale to the public by so-called small Indian chemists (of course excluding the reputable chemists of India) is not quinine at all but chalk. India is “par excellence” the dumping ground for every variety of quack medicine and adulterated drug manufactured in all parts of the world; its markets are glutted with useless and deleterious drugs sold by unqualified chemists who are themselves a public danger; and yet the Government looks on with complacency. I don't say that it does so deliberately, but that it has done so for many years there is no doubt. Otherwise it would have awakened to its responsibility years ago and introduced a Food and Drugs Act - a Pharmacy and Poisons Bill - and insisted on all Chemists being suitably qualified. ...”
He further spoke “...On the 9th March 1927 this matter was brought to the notice of the Council of State. I have before me that debate in which the Director General of the Indian Medical Service assured the Mover of the motion that he would represent to the various Provincial Governments with regard to the necessity of a Food and Drugs Act being introduced, as also that the training of pharmaceutists be placed on a proper footing. I understand that the various Governments have been consulted about this matter, but nothing material has been done for the past eighteen months and the millions of India are still dying of malaria. The Tropical School of Medicine in Calcutta has also pronounced its opinion about the danger of this drug adulteration and pressed for legislation. The Senior Trades Commissioner has also exposed these dangers and advised a Food and Drugs Act. Mr. J. C. Ghosh of Calcutta has also exposed these dangers and pressed for legislation. The Indian Chamber of Commerce in Bombay have also written to the Government of India, Education, Health and Lands Department, and asked for a Food and Drugs Act and standardisation of all drugs manufactured or imported into India. Representatives of well known drug manufacturers and the public Press have also widely ventilated the fraud and dangers. I ask the Government, what has it done? Nothing! when it was clearly its duty to act at once and so save India. Why has it failed to do its duty? Why has it not introduced a Food and Drugs Act- a Pharmacy and Poisons Bill and other remedial measures? I therefore ask the Government to give the House the following assurance, failing which; ask your permission to adjourn the House for further consideration of the matter. ...”
The motion was answered by Mr. G. S. Bajpai (Secretary, Department of Education, Health and Land). His full speech as reported officially [3] is quoted below:-

Although the Adjournment Motion was disallowed nonetheless, Government got enough indication that the crisis must be tackled seriously and addressed on priority basis. As a step forward in this direction Government of India, Department of Education, Health and Lands, approached all Local Governments vide letter No. 1908-H., dated the 7th November 1928 followed by another Letter No. 483-H., dated the 8th March 1929 [4] with following proposal:-
“The Government of India are of opinion that the most practical and satisfactory method of ascertaining how the problem should be dealt with would be to appoint a small ad hoc committee which would explore and define the scope of the problem with reference to actualities and make recommendations as to what steps, if any, are necessary to arrive at a satisfactory solution. The terms of reference of the Committee might be as follows:-
To enquire into the extent to which drugs recognized by the British Pharmacopoeia but of impure quality or defective strength are imported, manufactured and sold in British India, and the necessity, in the public interest, of controlling such importation, manufacture and sale; and to make recommendations.
As regards the personnel of the Committee, it has been suggested that two medical experts (of whom one should be the chairman), one representative of the drug trade, and one lay man to represent the consumer should be adequate. An officer with legal experience might be appointed Secretary to the Committee so as to provide the necessary legal assistance in suggesting draft of legislation, should the Committee decide to recommend legislation as part of the plan for dealing with the problem. The Government of India will be glad to be favoured with the views of the (Local) Government on the proposal to appoint a Committee and on its proposed terms of reference and personnel.”
Drugs Enquiry Committee
Finally on 11th of August 1930, the Government of India, Department of Education, Health and Lands, issued the following Resolution, appointing a committee:-
“No. 1637.- In pursuance of a Resolution which was adopted by the Council of State in March 1927 recommending the Governor-General in Council to urge all Provincial Governments to take such steps as may be possible to control the indiscriminate use of medicinal drugs and to legislate for the standardization of the preparation and for the sale of such drugs, the Government of India, after consulting and with the approval of Local Governments, have decided to appoint a small committee to explore and define the scope of the problem, and to make recommendations as to the measures which should be taken.
2. The terms of reference to the Committee will be as follows:-
(1) To enquire into the extent to which drugs and chemicals of impure quality or defective strength, particularly those recognized by the British Pharmacopoeia, are imported, manufactured or sold in British India, and the necessity, in the public interest, of controlling such importation, manufacture and sale and to make recommendations;
(2) To report how far the recommendations made in (1) may be extended to known and approved medicinal preparations other than those referred to above, and to medicines made from indigenous drugs and chemicals; and
(3) To enquire into the necessity of legislation to restrict the profession of pharmacy to duly qualified persons, and to make recommendations.
3. The Committee will be composed as follows:- Chairman.
(1). Lieut.Col. R. N. Chopra, M.A., M.D. (Cantab.), L.R.C.P. (London), M.R.C.S. (Eng·), I.M.S., Professor of Pharmacology. School of Tropical Medicine and Hygiene, Calcutta. Members.
(2). Rev. Fr. J. F. Caius, S.J., Pharmacologist at the Haffkine Institute, Bombay.
(3). Mr. H. Cooper, PH.C., F.C.S., of Messrs. Smith Stanistreet & Co. Ltd., Manufacturing Chemists, Calcutta.
(4). Maulvi Abdul Matin Chaudhury, M.L.A. A Secretary whose name will be announced later. [Mr. C. Govindan Nair, B.A., B.L., Barrister-at-Law, of the Madras Judicial Service, and Under Secretary to the Government of Madras, Law (Drafting) Department, was subsequently appointed Secretary.]
4. The Committee will have the power to co-opt members when necessary. It will visit important centres in the Provinces and will take evidence on the questions stated in the terms of reference. It will also issue a questionnaire to selected persons and bodies. Persons who desire to be called as witnesses or to receive copies of the questionnaire should apply in writing to the Secretary of the Committee, care of Lieutenant-Colonel Chopra, I.M.S., the Chairman of the Committee, School of Tropical Medicine and Hygiene, Calcutta, giving their full names and addresses, together with a brief memorandum of the points in regard to which they desire to give evidence. It will rest with the Committee to decide what oral evidence should be taken.”
Although, appointment of Secretary of the committee remained pending the enthusiastic Chairman Col R. N. Chopra put all resources at his disposal to instantly start the process of enquiry, in which he received immense support/assistance from Mr. H. Cooper who also happened to be from Calcutta.[5] A questionnaire in four parts having total 38 questions were prepared in consultation with all members and 2,180 questionnaire were issued during the month of September, 1930. The Secretary of the committee Mr. C. Govindan Nayar joined the team on 1 st October, 1930. Altogether 638 replies were received by the committee from different parts of British India as presented in table 1.
Table 1. Number of replies to questionnaire from different places
|
S. No. |
Presidency/Provinces/State etc. |
No. of reply |
|
1 |
Madras |
104 |
|
2 |
Bombay |
85 |
|
3 |
Bengal |
123 |
|
4 |
United Provinces |
90 |
|
5 |
Punjab |
68 |
|
6 |
Bihar and Orissa |
39 |
|
7 |
Central Provinces |
19 |
|
8 |
Assam |
16 |
|
9 |
Burma |
24 |
|
10 |
North-West Frontier |
11 |
|
11 |
Delhi |
11 |
|
12 |
Baluchistan |
02 |
|
13 |
Nepal |
02 |
|
14 |
Central India |
03 |
|
15 |
Ajmer-Merwara |
03 |
|
16 |
Indian States |
38 |
|
|
Total |
638 |
Out of the 638 replies 526 (82.44%) were from medical profession, 74 (11.59%) from Chemists and Druggists, and 38 (5.95%) from manufacturers. Moreover, 392(61.44%) replies were from officials and 246 (38.56%) were from non-officials.
The committee had co-opted 10 members from different regions as depicted below:

Details of the visits by the committee and witnesses examined during 17 th October, 1930 to 31 st January, 1931 are accounted in the following excerpt:-
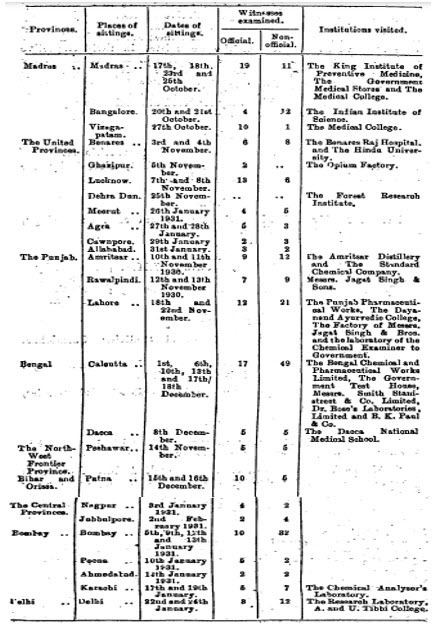
After completing the visits across British India and collecting evidence that began on 17 th October, 1930 at Madras and ended on 31 st January, 1931 at Allahabad, the committee compiled the voluminous 436 page report and submitted to Government of India on 29 th March, 1931. The post enquiry developments are available in author’s article published on 29 th March, 1923 [6] that eventually led to enactment of the Drugs Act, 1940.
Amendments
The very first amendment came in the wake of The Part B States (Laws) Act, 1951 (Act No. 3 of 1951) as depicted below. [7]

The Drugs (Amendment) Act, 1955 (11 of 1955) introduced wholesome changes [8] beginning with revising definition of ‘drug’ as depicted below:-

The original definition of ‘drug’ in the 1940 Act was as under:

The other changes made by the 1955 amendment were insertion of definition of ‘manufacturer’, amendment in section 5 of the Act to expand composition of Drugs Technical Advisory Board; amendments in sections 6, 10, 11, 12, 16, 18, 19, 22, 27, 28, 30, 33; substitution of section 34 of the Principal Act by new Chapter V Miscellaneous incorporating sections 34 to 37, and amendment in The Schedule to the Principal Act. Thus it was quite a substantive amendment expanding the original four chapters Act into five chapters Act.
The Drugs (Amendment) Act, 1960 (35 of 1960) once again revised the definition of ‘drug’, inserted definition of ‘Government Analyst’, ‘Inspector’ [9], substituted Sections 20, 21, 27, 30(1); amended sections 22, 23, 31, 33; and inserted Section 34 A. empowering Central Government to give directions to any State Government as may appear to the Central government to be necessary for carrying into execution in the State any of the provisions of this Act or of any rule or order made thereunder.
The complete details of the Drugs (Amendment) Act, 1962 (21 of 1962) [10] has been described in the preamble of this article and the readers can easily grasp its importance as it rechristened the Principal Act itself and expanded its spectrum of control over cosmetics in addition to drugs.
The Drugs and Cosmetics (Amendment) Act, 1964 (13 of 1964) further expanded the spectrum of control over Ayurvedic (including Siddha) and Unani drugs [11] by making appropriate alterations in sections 3, 4, 5, 6, 8, 10, 12, 16, 18, 19, 23, 25, 30, 31, 33, 33A, 36; inserting new sections 7A, 9B, 17B, 18A, 31A, 32A, 33A, 34A, 38; insertion of new Chapter IVA dedicated to provisions relating to Ayurvedic (including Siddha) and Unani drugs spread over sections 33B to 33O; substitution of section 27 covering penalty for manufacture, sale etc. of drugs in contravention of Chapter IV of the Act, and substitution of section 28.
The Drugs and Cosmetics (Amendment) Act, 1972 (19 of 1972) made a very important change in the Act by omitting the words “except the State of Jammu and Kashmir” in subsection (2) of section 1 of the Act and it [12] inserted new section 3A in the Drugs and Cosmetics Act. It was a brief but historic amendment of the Act.
The Drugs and Cosmetics (Amendment) Act, 1982 (68 of 1982) made several substitutions, omission and insertions in section 3. It [13] changed the heading of Chapter III and chapter IV; substituted sections 9; 9A and 9B by sections 9A, 9B, 9C and 9D; substituted section 13, 17, 17A, 17B, 27, 27A, 33D, 33E, 33I, 33J, 33M, 33N; amended sections 10, 12, 15, 18, 19, 21, 22, 23, 28,30, 31, 32, 32A, 33, 33C, 33F, 35, 36, 38; inserted new section 10A, 18B, 26A, 28A, 28B; 34AA, 36A; inserted sub-section (4) in section 20; amended the First Schedule and made transitory provisions for continuing the existing DTAB to function as DTAB for the Ayurvedic, Siddha and Unani drugs; until the constitution of the Ayurvedic, Siddha and Unani drugs DTAB.
The Drugs and Cosmetics (Amendment) Act, 1986 (71 of 1986) was once again a very brief amendment. It [14] amended sections 26 making provision for any recognised Consumer Association to get test or analysis done by the Government Analyst on payment of prescribed fee and receive report of such test or analysis. It also made commensurate amendment in section 32(1).
Section 83 of the Finance Act, 1952 (No. 22 of 1995) amended the Drugs and Cosmetics Act, 1940 as depicted below:-

This [15] occupies the distinction of being the briefest amendment as it only substituted the words “Custom Collector” by the words “Commissioner of Customs” in sub-section (1) of section 11 of the Drugs and Cosmetics Act, 1940.
The Drugs and Cosmetics (Amendment) Act, 1995 (22 of 1995) occupies the distinction of being the briefest amendment as it inserted only three words “Commissioner of Customs” in sub-section (1) of section 11 of the Act. The Drugs and Cosmetics (Amendment) Act, 2008 (26 of 2008) is the last in the series. It [16] inserted new section 17D, 26B, 32B, 33KA, 33KB, 36AB, 36AC, 36AD, 36AE; and amended sections 18, 26A, 27, 27A, 28, 28A, 29, 30, 32, 33, 33I, 33J, 33N, 36A of the Act.
The series of amendments as described in brief hereinabove explains how the nascent Drugs Act reached its zenith. Now the draft of new Drugs, Medical Devices and Cosmetics Bill, 2022 is in public domain. Once the new legislation comes the Drugs and Cosmetics Act, 1940 shall become history.
References
1. The Council of State Debates, (8th February 1927 to 29th March 1927), Third Session of the Second Council of State, 1927; Vol. 1: 9 th March 1927: Page 515-525.
2. J. W. D. Megaw, Sudhamoy Ghosh and N. R. Chatterjee. Stock Solutions of Quinine. The Indian Medical Gazette; Vol. LXIII, May 1928: 244-247.
3. The Legislative Assembly Debates, (Official Report) Volume III (4th September to 15th September 1928) Third Session of the Third Legislative Assembly. 4 th September, 1928; Motion for Adjournment-Quinine Fraud: Page 154- 157.
4. Report of the Drugs Enquiry Committee, 1930-31. Printed by The Superintendent, Government Press and Published by The Government of India, Central Publication Branch, Calcutta. Page 3-4. 5. Ibid. Pages 15-19.
6. Dr. R. S. Thakur, One Report Two Central Acts. PHARMATUTOR PHARMACY INFOPEDIA. 29th March, 1923, Reference Id: PHARMATUTOR-ART-3001.
7. The Part B States (Laws) Act, 1951 (Act No. 3 Of 1951) page 22. Available at https://legislative.gov.in/sites/default/files/A1951-3.pdf accessed on 02.04.2023.
8. The Drugs (Amendment) Act, 1955 (11 of 1955) Available at https://www.indiacode.nic.in/repealed- act/repealed_act_documents/A1955-11.pdf accessed on 02.04.2023.
9. The Drugs (Amendment) Act, 1960 (35 of 1960) Available at https://www.indiacode.nic.in/repealed- act/repealed_act_documents/A1960-35.pdf accessed on 02.04.2023.
10. The Drugs (Amendment) Act, 1962 (21 of 1962) Available at https://www.indiacode.nic.in/repealed- act/repealed_act_documents/A1962-21.pdf accessed on 02.04.2023.
11. The Drugs and Cosmetics (Amendment) Act, 1964 (13 of 1964) The Drugs and Cosmetics (Amendment) Act, 2008 (26 of 2008), w.e.f. 10-8-2009
12. The Drugs and Cosmetics (Amendment) Act, 1972 (19 of 1972). 13. The Drugs and Cosmetics (Amendment) Act, 1982 (68 of 1982) Received the assent of the President on Nov. 13, 1982 and published in the Gazette of India, Extra., Pt. II, Sec. 1, date November
13, 1982. Came into force on Feb. 1, 1983, vide G.S.R. 39(E), dated 25-1-1983.
14. The Drugs and Cosmetics (Amendment) Act, 1986 (71 of 1986). Notification No. G.S.R. 776(E), dated 15-9-1987, Gazette of India, Extraordinary, 1987, Part II, Section 3(i).
15. The Finance Act, 1952 (No. 22 of 1995). The Gazette of India Extraordinary Part II - Section 1, No.52 dated May 27, 1995. Page 24.
16. The Drugs and Cosmetics (Amendment) Act, 2008 (26 of 2008) Available at https://www.indiacode.nic.in/repealed-act/repealed_act_documents/A2008-26.pdf accessed on 02.04.2023."









