 About Authors:
About Authors:
Kapil Patel, Supriya Hazra Das
Department of Pharmacology
Shree Dhanvantary Pharmacy College,
Kim, Surat – 394118,
Gujarat, India
Abstract:
Peptic ulcer is a condition where benign lesions of gastric or duodenal mucosa occur at a site where the mucosal epithelium is exposed to acid and pepsin, due to imbalance between offensive and defensive factors. Acacia leucophloea, a deciduous tree found throughout India is reported to be used in gastric ulcer in traditional medicine. The present study was undertaken to evaluate the anti-ulcer properties of Acacia leucophloea in pylorus ligation ulcer model in rats. Acacia leucophloea was administered for 7 days and on 8th day 30 min prior to the induction of ulcers. Pretreatment with Acacia leaucophloea showed significant ulcer protection against pylorus ligation ulcer model as compared to control. Thus, the ulcer protective and healing effects of Acacia leucophloea may be due to its effect on both offensive and defensive factors. The present findings suggest that Acacia leucophloea has antiulcer properties.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1223
Introduction:
Peptic ulcer disease is a serious gastrointestinal disorder that requires a well targeted therapeutic strategy. A number of drugs including proton pump inhibitors and H2 receptor antagonists are available for the treatment of peptic ulcer, but clinical evaluation of these drugs has shown incidence of relapses, side effects, and drug interactions. This has been the rationale for the development of new antiulcer drugs and the search for novel molecules has been extended to herbal drugs that offer better protection and decreased relapse. Drugs of plant origin are gaining popularity and are being investigated for a number of disorders, including peptic ulcer
Acacia leucophloea is a plant from the family fabaceae, is extensively cultivated in some regions of the world. The bark of Acacia leucophloea is commonly used as an anti-inflammatory, in skin diseases, in leucoderma, in diarrhea, also in dental caries.
Materials and Method:
Materials
Anesthetic ether
Sodium hydroxide
Ranitidine
Methanol
Topfer's reagent
Plant material
Barks of Acacia leucophloeawere collected from surrounding areas of Surat district, Gujarat, and authentication of the plant was done by Dr. Bimal Desai, Professor, Department of Botany, Agriculture University, Navsari. The collected material was washed with running water. The barks were chopped in to small pieces and dried under shade. Dried barks were coarsely powdered and used for extraction.
METHODOLOGY
Preparation of Extracts
Authenticated barks of acacia leucophloeawere shade dried and pulverized in to coarse material. Coarse plant material was cleaned by passing the powder material through 120 mesh sieve to remove any fine dust or powder, and coarse powder was used for extraction. Dried powder of bark was exhaustively extracted using Methanol (90%) (AME) by maceration method. The extract was concentrated by rotary flash evaporator, under reduced pressure and controlled temperature, followed by freeze drying and stored in a desiccator.
Experimental animals
Female Wister rats weighing 150-170g was used for the present study. The animals were purchased from Shree Dhanvantary Pharmacy College, Kim, Surat. They were maintained in the animal house of Shree Dhanvantary Pharmacy College for experimental purpose. The animals were maintained under controlled conditions of temperature (23 ± 2°C), humidity (50 ± 5%) and 12-h light-dark cycles. All the animals were acclimatized for seven days before the study. The animals were housed individually in sanitized polypropylene cages containing sterile paddy husk as bedding. They had free access to standard pellets as basal diet and water ad libitum.Animals were habituated to laboratory conditions for 48 hours prior to experimental protocol to minimize if any of non-specific stress. All the studies conducted were approved by the Institutional Animal Ethical Committee (IAEC) of Shree Dhanvantary Pharmacy College, Kim, Surat, Gujarataccording to prescribed guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
[adsense:468x15:2204050025]
Anti Ulcer activity in Pylorus ligation in rats
Group I (control): Untreated.
Group II (standard): treated with Ranitidine (20 mg/kg,).
Group III (AME 200): treated with AME (200 mg/kg,).
Group IV (AME 400): treated with AME (400 mg/kg,).
The animals were fasted for overnight before pylorus ligation with water ad libitum. Under ether anesthesia, the abdomen was opened by midline incision process. The pyloric portion of the stomach was slightly lifted out and ligated without damage to the blood supply. AME was administered before pylorus ligation. The stomach was placed back carefully and abdominal wall was closed by sutures. Animals were sacrificed by an over dose of anesthetic ether after four hours of pylorus ligation. The stomachs were isolated and the content of the stomachs were collected and centrifuged at 2000 rpm for 10 min. The volume of the gastric juice was measured and this was used for the determination of pH, free acidity and total acidity. The ulcer index (U.I) and % Inhibition of Ulceration were determined.
Determination of pH
An aliquot of 1 ml gastric juice was diluted with 1 ml of distilled water and pH of the solution was measured using pH meter.
Determination of free acidity
Instead of phenolphthalein indicator, the Topfer's reagent was used. Aliquot of gastric juice was titrated with 0.01N NaOH until canary yellow color was observed. The volume of 0.01N NaOH consumed was noted. The free acidity was calculated by the same formula for the determination of total acidity.
Determination of total acidity
An aliquot of 1ml gastric juice diluted with 1ml of distilled water was taken into a 50 ml conical flask and two drops of phenolphthalein indicator was added to it and titrated with 0.01N NaOH until a permanent pink color was observed. The volume of 0.01N NaOH consumed was noted. The total acidity is expressed as meq./l by the following formula:
Acidity = n Χ 0.01 Χ 36.45 Χ 1000
Where,
n is volume of NaOH consumed,
36.45 is molecular weight of NaOH,
0.01 is normality of NaOH,
1000 is the factor (to be represented in liter).
Macroscopic evaluation of stomach
The stomachs were opened along the greater curvature, rinsed with saline to remove gastric contents and blood clots and examined by a Χ5 magnifier lens to assess the formation of ulcers. The numbers of ulcers were counted. Ulcer scoring was undertaken according to Vogel et al.
The scores were:
0 = no ulcer | 1 = superficial ulcer | 2 = deep ulcer | 3 = perforation
Ulcer index was measured by using following formula according to vogel et al.
Ulcer Index (U.I)= UN + US + UP Χ 10-1
Where,
UN= Average number of ulcers per animal
US =Average number of severity score
UP=percentage of animals with ulcers
Percentage inhibition of ulceration was calculated as below:
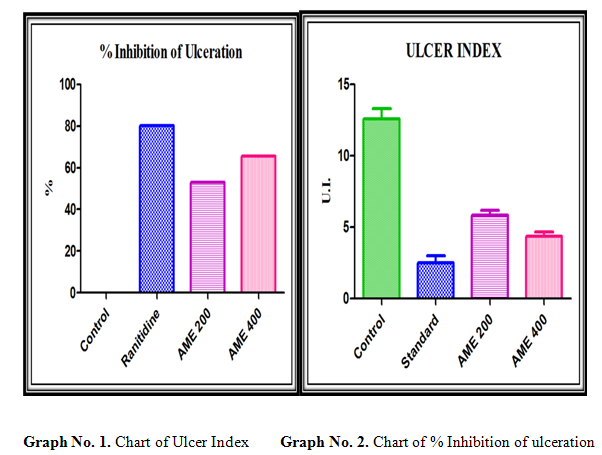
% Inhibition of Ulceration= (U.I Control – U.I Test) Χ 100 / U.I Control
Statistical analysis
The values are represented as the mean±SEM for each group. Statistical signification between treated and control groups were analyzed using a One-way analysis of variance (ANOVA) followed by Dennett’s t-test. Results were considered to be statistically significant at P<0.001.
Results:
Table No. 1. Ulcer index of Control rats (4 hrs pylorus ligation)
|
Sr. No. |
Body weight |
Ulcer index |
% Inhibition of Ulceration |
|
1. |
150 |
15.5 |
0% |
|
2. |
175 |
11.5 |
|
|
3. |
150 |
12 |
|
|
4. |
155 |
13.5 |
|
|
5. |
170 |
10.5 |
|
|
6. |
160 |
12.5 |
Table No 2. Ulcer index of Ranitidine treated rats (4 hrs pylorus ligation)
|
Sr. No. |
Body weight |
Ulcer index |
% Inhibition of Ulceration |
|
1. |
160 |
2.5 |
80.13% |
|
2. |
175 |
3.0 |
|
|
3. |
175 |
4.0 |
|
|
4. |
180 |
2.0 |
|
|
5. |
150 |
0.5 |
|
|
6. |
150 |
3.0 |
Table No. 3. Ulcer index of 7 days AME 200 treated rats (4 h pylorus ligation)
|
Sr. No. |
Body weight |
Ulcer index |
% Inhibition of Ulceration |
|
1. |
150 |
5.0 |
52.98% |
|
2. |
175 |
6.5 |
|
|
3. |
170 |
6.0 |
|
|
4. |
175 |
7.5 |
|
|
5. |
180 |
5.0 |
|
|
6. |
150 |
5.5 |
Table No. 4. Ulcer index of 7 days AME 400 treated rats (4 h pylorus ligation)
|
Sr. No. |
Body weight |
Ulcer index |
% Inhibition of Ulceration |
|
1. |
150 |
3.5 |
65.56% |
|
2. |
175 |
5.0 |
|
|
3. |
170 |
4.5 |
|
|
4. |
175 |
5.5 |
|
|
5. |
180 |
3.5 |
|
|
6. |
150 |
4.0 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Antacid activity
Table No. 5. Acid volume, pH, free acidity, total acidity of Control rats
|
Sr. No. |
Body weight |
Vol. of gastric juice in ml/100g body weight |
pH |
Free acidity meq/l/100g |
Total acidity meq/l/100g |
|
1 |
150 |
4.05 |
2.5 |
39 |
86 |
|
2 |
175 |
4.10 |
2.3 |
45 |
87 |
|
3 |
150 |
3.80 |
2.1 |
41 |
91 |
|
4 |
155 |
3.50 |
2.5 |
43 |
94 |
|
5 |
170 |
3.40 |
2.2 |
38 |
88 |
|
6 |
160 |
3.65 |
2.5 |
44 |
92 |
|
Mean ± SEM |
- |
3.800±0.106 |
2.350±0.072 |
41.667±1.14 |
89.67±1.282 |
Table No. 6. Acid volume, pH, free acidity, total acidity of Ranitidine treated rats
|
Sr. No. |
Body weight |
Vol. of gastric juice in ml/100g body weight |
pH |
Free acidity meq/l/100g |
Total acidity meq/l/100g |
|
1 |
160 |
1.75 |
4.1 |
20 |
47 |
|
2 |
175 |
1.95 |
4.2 |
18 |
45 |
|
3 |
175 |
2.10 |
4.5 |
17 |
48 |
|
4 |
180 |
2.00 |
4.2 |
18 |
43 |
|
5 |
150 |
1.85 |
4.1 |
19 |
41 |
|
6 |
150 |
2.00 |
4.3 |
22 |
40 |
|
Mean ± SEM |
- |
1.942±0.051 |
4.233±0.061 |
19.00±0.730 |
44.000±1.317 |
Table No. 7. Acid volume, pH, free acidity, total acidity of AME200
|
Sl. No. |
Body weight |
Vol. of gastric juice in ml/100g body weight |
pH |
Free acidity meq/l/100g |
Total acidity meq/l/100g |
|
1 |
150 |
3 |
5.5 |
30 |
72 |
|
2 |
175 |
3.1 |
5.6 |
28 |
69 |
|
3 |
170 |
3 |
5.5 |
34 |
65 |
|
4 |
175 |
2.8 |
5.4 |
28 |
63 |
|
5 |
180 |
3.1 |
5.7 |
33 |
69 |
|
6 |
150 |
2.9 |
5.8 |
35 |
71 |
|
Mean ± SEM |
- |
2.983±0.048 |
3.367±0.082 |
31.333±1.256 |
68.167±1.424 |
Table No. 8. Acid volume, pH, free acidity, total acidity of AME400
|
Sr. No. |
Body weight |
Vol. of gastric juice in ml/100g body weight |
pH |
Free acidity meq/l/100g |
Total acidity meq/l/100g |
|
1 |
150 |
2.3 |
4.0 |
26 |
55 |
|
2 |
175 |
2.5 |
3.6 |
24 |
52 |
|
3 |
170 |
2.4 |
3.5 |
29 |
58 |
|
4 |
175 |
2.3 |
3.4 |
23 |
56 |
|
5 |
180 |
2.5 |
3.7 |
28 |
53 |
|
6 |
150 |
2.4 |
3.8 |
30 |
57 |
|
Mean ± SEM |
- |
2.400±0.037 |
3.667±0.088 |
26.667±1.145 |
55.167±0.946 |



Discussion:
The results in pyloric ligation model showed significant reduction in basal gastric secretion and inhibition of ulcers by Acacia leucophloea. This suggests that the antiulcer activity of Acacia leucophloea on gastric mucosa may be due to the reduction of gastric secretion through one or more of the possible mechanisms.Moreover, gastric acid is an important factor for the genesis of ulceration in pyloric ligation ulcer in rats. Gastric acid secretion is regulated by many factors including anxiety, vagal activity, cholinergic, histaminergic and gastrinergic neurotransmissions, the activities of various post-synaptic receptors and the proton pump. It is therefore, difficult to elucidate the relationship between the mechanisms of inhibition of gastric acid by Acacia leucophloea.
The antiulcer property of A.leaucopholea in pylorus ligation model is evident from its significant reduction in free acidity, total acidity, number of ulcers and ulcer index. A.leaucopholea treated animals significantly inhibited the formation of ulcers in the pylorus ligated rats and also decreased both the concentration and increased the pH, it is suggested that. A.leaucopholea can suppress gastric damage induced by aggressive factors.
The current data clearly demonstrated that Acacia leucophloea inhibited the aggressive factor, gastric acid secretion. The anti-ulcerogenic effect of the Acacia leucophloea may be related to its antisecretory action since acid is a major factor in the development of peptic ulcer.
The current data also clearly demonstrated that the 400 mg/kg are more effective than the 200 mg/kg dose of AME.Acacia leucophloea has shown increased pH and decreased total acidity of gastric fluid.
Conclusion:
We conclude that the methanol (AME 200) and methanol (AME 400) extract of Acacialeucophloeabark has potent antiulcer activity in rats. The present investigation has also opened avenues for further research especially with reference to the development of potent phytomedicine for treatment of anti ulcer from the title plant.
Bibliography:
1. Valle DL. Peptic ulcer diseases and related disorders. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 16th ed. New York: McGraw-Hill; 2005. p. 1746-62.
2. Hoogerwerf WA, Pasricha PJ. Agents used for control of gastric acidity and treatment of peptic ulcers and gastro esophageal reflux disease. In: Hardman JG, Limbird LE, Goodman Gilman A, editors. Goodman and Gilman, The Pharmacological Basis of Therapeutics. 10th ed. New York: Mc Graw-Hill; 2001. p. 1005-19.
3. J Ravikumar, H Y Santosh, M N Nagashruthi, Antiulcer activity of Anisochilus carnosus leaf extract, undefined, vol 45, issue 12, 979.
4. Gerhard Vogel H. Drug Discovery and Evaluation Pharmacological Assays. 2nd ed. New York: Springer - Verlag Berlin Heidelberg. 2002. p. 867-8.
5. Aguwa CN, Mittal GC. Study of antiulcer activity of aqueous extract of leaves of Pyrenacantha standtii (Family I cacinaceae) using various models of experimental gastric ulcer in rats. European Journal of Pharmacology 1981; 74: 215-9.
6. Brodie D.A. The mechanism of gastric hyperacidity produced by pylorus ligation in the rat. Am. J. Dig. Dis. 11 (3): 231-241 (1966).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









