About Authors
Rajora Priyanka Singh*, Rathore Kamal Singh
Faculty of Pharmacy, BN University, Udaipur-Raj.313001
*singhpriyanka20355@gmail.com
ABSTRACT
Lyophilization or freeze drying is a process in which water is remove from the product through the process of sublimation and desorption after it is frozen. It is most common method for manufacturing of product which are unstable in liquid form. this method is divided in the three steps these are freezing, primary drying and secondary drying. SMARTTM freeze dryer technology is same like a freeze-drying technology but this technology is a primary drying optimization tool which work on manometric temperature measurement (MTM) technique, a pressure rise measurement technique. This technique is used to determine the cake resistance and product temperature at the ice interface among other parameters. AutoMTM is another mode of operation of the SMART Freeze dryer technology and this mode allows the researchers to run their own pre-determined cycle but too collect the critical process data and product parameters calculated by SMART. This technology is a further valuable tool to optimize an existing freeze-drying process.
INTRODUCTION
Lyophilization or Freeze drying is a process in which water is frozen, followed by its removal from the sample, initially by primary drying (sublimation) and then by secondary drying (desorption). The term “lyophilization” describes a process to supply a product that “loves the dry state”. Drying from the frozen state is common in nature (Example- In the winter, snow vanishes on the roads in dry cold air without melting, this is also the process of freeze drying). Freeze drying is a process that is widely used for pharmaceuticals or food products which are thermolabile in nature.it is the most common method for parenteral for which solution stability is an issue. Freeze drying is widely used in the pharmaceutical industry because the low operating temperatures reduce the damages which can occur with traditional drying processes that use higher temperatures so the quite expensive pharmaceuticals allows the use of this technology due to the slow drying rate, the use of vacuum, and the high investment and operating costs. Freeze drying is the removal of water or other solvents from a material by the process of sublimation and the removal of bound water molecules by the process of desorption. Lyophilization and freeze drying are terms interchangeably depending on the industry and location where this process is happened. freeze drying maintain the product temperature low enough during the process to avoid the changes in final product. This is the best method to preserve the wide variety of heat sensitive materials. Other uses of this technique including the stabilization of living materials (for example microbial cultures, preservation of whole animal specimens for museum display, restoration of books and other items damaged by water, and therefore the concentration and recovery of reaction products). This method is more suitable for proteins and peptides because they are degraded on higher temperatures. (1, 2)
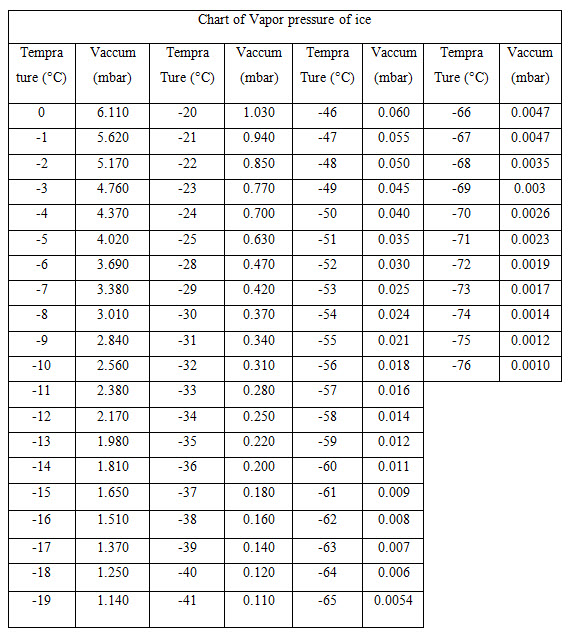
The main principle involved in freeze drying is a sublimation, in which water passes directly from solid state (ice), to vapor state without passing through the liquid state if the ambient partial water vapor pressure is less than the partial pressure of the ice at its relevant temperature. A scientific way to determine an appropriate pressure for freeze drying is to choose a system pressure that is 20% To 30% of the vapor pressure of ice at the target product temperature so This process take place at pressures and temperature below the triple point of water. (3, 4)
Table 1 : Vapor pressure of ice
The steps required to lyophilize the product can be summarized as follows:(5,6)
1. Pretreatment / formulation
2. Loading
3. Freezing
4. Primary drying
5. Secondary drying
6. Backfill and stoppering
7. Removal of dried product from freeze dryer
Pretreatment includes the dissolving drugs and excipients in the solvent, then sterilize this solution by passing it through bacteria retentive filter and then filling of this solution into the container. Pretreatment includes any method of treating the product (changes in formulation) before freezing. this is done to reduce the cycle time of freeze drying. Then load this solution into the freeze dryer. Then cycle is started by the process of freezing. Freezing is very important that the sample be fully frozen prior to pulling a vacuum and starting the drying process. Freezing method maximizes the product surface area and minimizes its thickness for efficient water removal from the sample. A cooling rate of about 1°C/min yields moderate supercooling with moderate ice surface area and a reasonably fast freezing rate and produces uniform ice crystals. Annealing is an optional step in which the product is hold at a temperature above the final freezing temperature for defined period to crystallize the crystalline components in the formulation during freezing stage. It produces the uniform size crystals. Then process of primary drying is start. in the primary drying, bulk of water removed from the product during freeze drying is via sublimation of all of the free ice crystals. Sublimation is the slow process conducted at the lower temperature, below the product critical collapse temperature. Sublimation requires heat energy to change the phase from solid to gas. In addition to the free ice that is sublimed during primary drying, then remaining water molecules that are bound to the product are removed by desorption (secondary drying). All of free ice has been removed in primary drying, the product temperature can now be increased considerably without fear of melting or collapse for secondary drying. Secondary drying actually starts during the primary phase, but at elevated temperatures (30°C to 50°C), desorption proceeds much more quickly. Secondary drying rates are dependent on the product temperature.
There are three methods (manifold drying, batch drying and bulk drying) used for freeze drying. Each method features a specific purpose, and therefore the method used depends on the product and the final configuration desired. The main components of freeze-drying equipment are:
1. Refrigeration system
2. Vacuum system
3. Control system
4. Product chamber or manifold
5. Condenser
6. Shelves
7. Shelf fluid system
8. Sensor
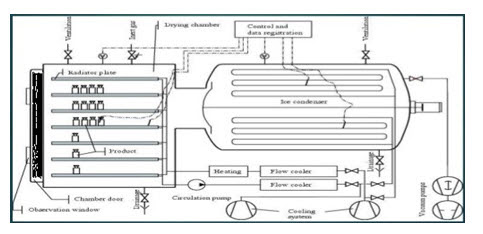
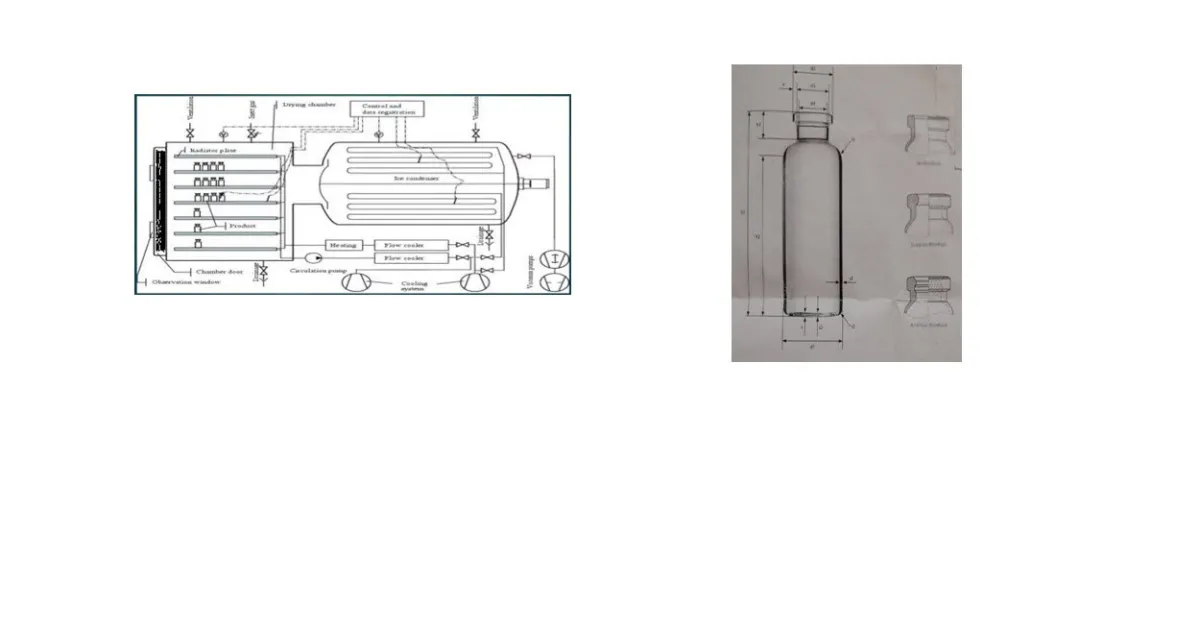
Figure1: Basic construction of freeze drying
Basic construction of all freeze dryers is same but SMARTTM freeze dryer automatically set the cycle and, in other dryer cycle is set by scientist. Refrigeration system cools the condenser located in the freeze dryer. Vacuum system consists of a separate vacuum pump connected to a condenser and attached product chamber. Along with condenser system it provides necessary pressure for conducting primary and secondary drying processes. Control systems vary in complexity and typically include temperature and pressure sensing ability. Controllers will allow the programming of an entire “cycle” for freeze drying and can include options to watch how the freeze-drying process is progressing. Product chambers are either a manifold with attached flasks, or, an outsized chamber with a system of shelves on which to put the product. this chamber performs two functions:
1. To provide a safe environment for a product during lyo cycle.
2. To provide the necessary temperature and pressure to conduct entire process
Condenser attract the vapours being sublimed off of the product. Because condenser is maintained at a lower energy level relative to the product ice, the vapours condense and turn back into ice in the condenser. Shelves are most important of freeze dryer. The shelf act as a heat exchanger, removing energy from the product during freezing, and supplying energy to the product during primary and secondary drying of the freeze-drying cycle. These shelves are going to be connected to the silicone oil system through either fixed or flexible hoses and it are often manufactured in sizes up to 4 m2 in area. Shelf fluid system circulating a fluid (silicone oil) through the shelves at a desired temperature for energy exchange in freeze drying cycle. Sensor is important to measure the temperature and pressure during entire process of freeze drying. Temperature is measured by the thermocouples and other method whereas pressure is measured by Pirani guages. SMARTTM freeze dryer has same construction but it automatically set the freeze-drying cycle on the basis of parameter which are entered in this system. (6,7)
Advantages: (8)
1. No stability problem
2. Good for O2 and air sensitive, temperature sensitive drugs.
3. Raped constitution time
4. Also use to increase the shelf life of some pharmaceuticals for many years.
Disadvantages: (8)
1. Expensive
2. Sterility problems
3. Volatile compounds may be removed by high vacuum
4. If too much heat is added, the material’s structure could be altered.
SMARTTM Freeze Dryer Technology
SMARTTM Freeze dryer technology is primary drying optimization tool which work on manometric temperature measurement (MTM) technique, a pressure rise measurement technique. This technique is used to determine the cake resistance and product temperature at the ice interface among other parameters. SMART eliminates the trial and error approach normally used to develop new cycle quickly, while ensuring product quality, efficiency and robust process. the illustration depicts a return of investment on how the SMART technology can reduce the development time. AutoMTM is another mode of operation of the SMART Freeze dryer technology and this mode allows the researchers to run their own pre-determined cycle but also collect the critical process data and product parameters calculated by SMART. This technique is a further valuable tool to optimize an existing freeze-drying process. (9)
SMARTTM Freeze dryer technology is a primary drying optimization tool which work on manometric temperature measurement (MTM) technique, a pressure rise measurement technique and it eliminates the trial and error approach normally utilize to develop the cycle quickly. It ensures the graceful transfer from laboratory to production scale. major challenges in freeze drying are the development of an optimized lyophilization cycle, and scale-up of the freeze-drying cycle from a laboratory to pilot or production scale unit. Understanding the characteristics of the product and therefore the lyophilizer performance are crucial for successful freeze drying. Critical process parameters in developing a freeze-drying cycle include the collapse temperature of the formulation, the stability of active pharmaceutical ingredient and the properties of the excipients. Accelerating and streaming of lyo cycle is completed by SMARTTM freeze-drying technology. This technology reduces the average cycle development process to at least one or two runs instead of traditional cycle series of six to eight runs and it also reduces material cost. The principle behind the technology is that the manometric temperature measurement which delivers an accurate calculation of the product temperature at the sublimation interface, without having to put thermocouples or other temperature sensors in product vials. With the MTM technique, an isolation valve is placed between the product drying chamber and therefore the freeze dryer condenser. Input parameters before to running a SMARTTM freeze-drying cycle include:
1. Number of vials: Enter the total number of vials containing product in batch. A minimum number of vials is necessary to generate the accurate data.291cm2 of product surface area is required. This equates to approximately one third to one half of one tray of vials.
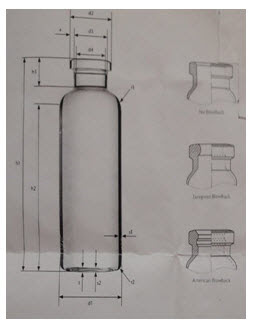
Figure 2: Vials used in lyophilization
2. Inner area of vials (Ap): The surface area of inner bottom surface of the vial in square centimetres (cm2). This information can be obtained from vial supplier or calculated from vial drawings using formula, A=∏2 r2, where r= the inner vial radius. The vials used should all have the same surface area. E.g. A common brand of 5 cc vial has 2.91cm2 inner area. A 20 cc vial has approximately 5.82 cm2.
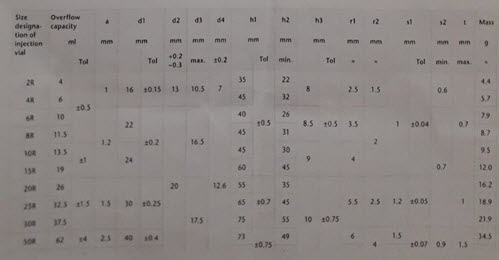
Figure 3: Different vial area
3. Fill Volume (cc): The fill volume of the solution in one vial in cubic centimetres. The fill volume should be the same for each vial in the batch.
4. Fill Weight (grams): The net weight of a single vial’s fill volume in grams. The fill weight should be the same for each vial in batch.
5. Teu, Tg’ or Tc: Teu: eutectic temperature of the formulation (°C)
Tg: Glass transition temperature of the formulation (°C)
Tc: Collapse temperature of the formulation (°C)
Input the appropriate parameter depending upon the nature of the product. Where more than one value exists, the lowest value should be used for the safest cycle. Freeze dry microscopy (FDM) is the best method to obtain this value. Differential scanning calorimetry (DSC) is also used.
6. Concentration of Solution: The amount of solute per unit amount of solution (g/g).
7. MTM Interval (minutes): This value will determine how often the isolation valve is closed during primary drying to perform the pressure rise and manometric temperature measurements (MTM).
8. Nature of drug Product: For Information only, select either a protein or small molecule depending on the type of product. This information will be included in SMART data file that is generated during the cycle.
9. Type of vials: Enter type of glass vial being used: tubing or molded vials. Tubing vials are generally recommended for lyophilization applications. This parameter will determine the heat transfer coefficient used by the program.
10. Type of bulking agent: Select the appropriate value according to the type of bulking agent being used (none, crystalline and amorphous). This selection will determine the default freezing program, default upper shelf set point limit in primary and ramp rates and shelf set points in secondary drying.
If crystalline is selected, there will be an annealing step (raising the shelf set point just above the critical temperature for 4 hours and then returning to a shelf set point of -40°C) and the system will ramp into secondary drying at the rate of 0.3°C /minute. The secondary drying shelf set point will have two segments- at +40°C and +50°C. If amorphous is selected, there will not be an annealing step during the freezing phase, the system will ramp into secondary at the rate of 0.1°C/minute and the shelf set point during secondary drying will be +40°C.
11. Upper shelf limit: It is recommended that the upper shelf set point limit during primary drying be +5°C for amorphous products and +25°C for crystalline products. These are the system defaults. Use this field to increase or decrease these limits.
12. End Point Pressure Sensitivity: In order to step into Secondary Drying, the program monitors the calculated Pice against the chamber pressure set point. When the vapour pressure of ice registers less than the end point pressure sensitivity value for two consecutive MTMs, the program will advance to the secondary drying phase.
13. Last Step for MTM Collection: When using the Auto-MTM mode of operation, it may not be necessary to perform MTM in every step of the drying program. Use this field to program the last step of recipe for which MTM is required.
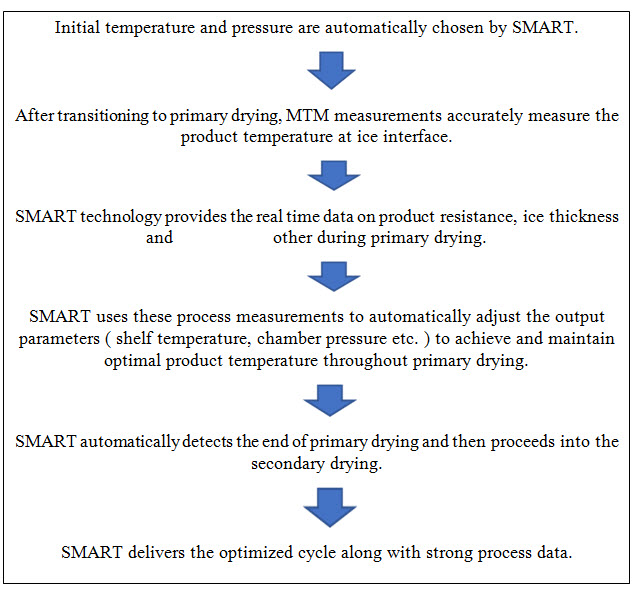
Figure 4: SMARTTM freeze dryer technology
During this cycle, the isolation valve is quickly and mechanically closed, and thus the rise in pressure is measured for 25 seconds at systematic intervals during primary drying. The data is accumulated and utilized in MTM equation to calculate the product temperature at the ice surface interface, the dried layer resistance, the ice thickness, and therefore the heat flow and mass transfer. This information is then applied to automatically adjust the Condenser Temperature, Shelf inlet temperature, Shelf setpoint, Product probe, Chamber CM, Chamber PIRANI, Condenser vacuum, Vacuum setpoint of the lyophilizer during freeze drying, thus achieving and maintaining the product temperature precisely at the target temperature throughout the freeze-drying cycle. A minimum product surface area of greater than 300 square centimetres or three-quarters of a sample tray is required to get good MTM data. (9)
Conclusion
Freeze drying is a process in which frozen water is removed from the product by the process of sublimation and then desorption. Smart freeze dryer also does the same process but its work on MTM principle aby eliminating the trial and error approach. MTM equations measure the product temperature and pressure without use of thermocouples automatically. This equation measures these parameters on the basis of input parameters which are feed in it. Then SMART uses the process measurement to automatically adjust the output parameters to achieve the optimized cycle. then SMARTTM automatically detects the end of primary drying and proceeds into the secondary drying. SMART removes the wastage of time in set the cycle of freeze drying.
References
1. Bhambre Deepak, Gaidhani A Kunal, Harwalkar Mallinath, Nirgude S Pallavi “Lyophilization/ freeze drying – A Review” “World Journal of Pharmaceutical Research” 2015, 4(4), 516-543.
2. Kumar Sandeep, Gautam Namrata, Kapoor Sapna, “Development and Optimization of Lyophilization cycle” “World Journal of Pharmaceutical Research” 4(2), 1053-1062.
3. Khairnar Sandip, Kivi Rajesh, Harwalkar Mallinath, Salunkhe Kishor “A Review on Freeze Drying Process of Pharmaceuticals” “International journal of Research in Phrmacy”2013, 4(1),76-94.
4. GR. Nireesha, L Divya, C Sowmya, N Venkateshan, Niranjan Babu, V Lavakumar “Lyophilization/Freeze Drying-An Review” “International Journal of Novel Trends in Pharmaceutical Sciences”2013, 3(4), 87-98.
5. Jadhav R Tushar, Moon RS, “Review on lyophilization technique”, “World Journal of Pharmaceutical Sciences”, 2015,4(5),1906-1928.
6. Barley John, “Basic Principles of Freeze Drying” SP Scientific.
7. Bisht Deepak, Dr. Iqbal Zeenat, “Lyophilization-Process and Optimization for Pharmaceutics” “International Journal of Drug Regulatory Affairs” 2015, 3(1), 30-40.
8. Shukla Soham “Freeze Drying Process: A Review” International Journal of Pharmaceutical Sciences and Research” 2011, 3(2), 3061-3068.
9. Mather Leslie, Director of Pilot Freeze Dryers, “Smart Freeze Dryer” SP Scientific.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE