About Authors:
M. B. Shinde*, S.V.Usnale1, M. B. Sorde2, O.V. Kolgire3
Department of quality assurance,
Maharashtra College Of Pharmacy, Nilanga.
Dist- latur (MS)
*maheshinde7@gmail.com
Abstract:
This paper reviews nanoparticles designed and prepared for cancer therapy and diagnosis. This review provides an update of tumor targeting with conventional or long-circulating nanoparticles. The mechanisms of cancer therapy by nanoparticles are explained in detail with their types. Photodynamic cancer therapy and photo thermal therapy are used for the cancer treatment by nanoparticles. There are two processes of nanoparticles on photodynamic cancer therapy these are active and passive. In photo thermal therapy there are diverse range of nanoparticles such as, Gold–Silica Nanoshells, Gold Nanorods, Small NIR-Tunable Gold Nanoparticles, GoldColloidal Nanospheres and Selecting a Therapeutic Gold Nanoparticle for Translation The synthesis of gold nanoparticles is explained in this article. The role of gold nanoparticles such as colloidal gold, gold nanorods, nanodumbbells, and nod-shaped gold particles are used for the cancer treatment.
REFERENCE ID: PHARMATUTOR-ART-1891
Introduction:
In 2010, the National Institutes of Health estimates that more than 1.5 million new cases of cancer will have been diagnosed in the United States, with an overall projected cost of US$263.8 billion. The development of new approaches to improve screening, diagnosis, and treatment of cancer is an area of intensive research spending and has generated numerous innovations that have enhanced the 5-year survival rates of cancer patients. However, these new treatments also contribute to the rising costs of healthcare. To justify these increased costs, state-of-the-art treatments should have enhanced efficacy, decreased invasiveness, and fewer side effects than current cancer therapies. While still in a relatively early stage of technological development, gold-nanoparticle based diagnostic and therapeutic approaches, particularly those based on gold-nanoparticle-mediated hyperthermia, have shown promise towards achieving these goals.
Nanoparticle contrast agents offer the potential to significantly improve existing methods of cancer diagnosis and treatment.
Figure 1. Gold nanoparticles
Advantages include biocompatibility, selective accumulation in tumor cells, and reduced toxicity. Considerable research is underway into the use of nanoparticles as enhancement agents for radiation therapy and photodynamic therapy, where they may be used to deliver treatment agents, produce localized enhancements in radiation dose and selectively target tumor cells for localized damage.
Gold is a favoured material for the cancer treatment. Formed into spheres, shells, cages or wires on the scale of ten to one thousand nanometres, gold can be bound to a wide variety of biochemically functional groups and made to target specific types of cells. Once there, the nanoparticles may be used for imaging or, in conjunction with an external energy source such as an infrared laser, to concentrate lethal doses of energy in the target cancer cells. Many re searchers believe that nanoparticle-based techniques could prove to be more effective than current chemical- or radiation-based treatments, with fewer adverse side effects.
Nanoparticle-based techniques have the potential to over many advantages over more conventional forms of cancer treatment. At present, the most common cancer treatment methods are based on chemotherapy, radiation therapy, or surgery, all of which can be successful, but have substantial disadvantages. Chemotherapy often induces severe side effects and can cause damage to healthy cells, and radiation is only useful on localized, well-defined tumours. Surgical removal also requires that the tumour be well localized, and is often impossible if the tumour is surrounded by sensitive tissues such as the brain.
Synthesis- Gold nanoparticles are produced in a liquid by reduction of chloroauric acid. After dissolving this acid, the solution is rapidly stirred while a reducing agent is added. This causes aluminium ions to be reduced to neutral gold atoms. As more and more of these gold atoms form, the solution becomes supersaturated & gold gradually starts to precipitate in form of sub-nanometer particles. Properties and applications of colloidal gold nanoparticles depends upon shape, e.g.; - rod like particles have both transverse and longitudinal absorption peak & anisotropy of shape affects their self-assembly.
Mechanism:
Since the development of medical lasers, scientists have been turning to electromagnetic radiation to treat cancer. This can be done in several ways. Photodynamic therapy (PDT) involves a photosensitizer which becomes excited and generates singlet oxygen and other free radicals. The combination of the generated heat and the interaction of the cells with the oxygen cause irreparable damage to the cancerous cells.
This method requires short wavelengths of light, reducing the depth to which the light can penetrate and making deep tumour treatment un- achievable. Another method of cancer treatment using electromagnetic radiation is photothermal therapy (PTT). Using this method, near infrared (NIR) radiation from a laser may be used to penetrate through the skin to a deeper extent because the light will undergo less absorption from tissue chromophore and water. Heat absorbed from the radiation will cause thermal denaturation and coagulation of affected cells. In addition, heating will cause vaporization of surrounding uids, producing cavitation bubble formation and what may be compared to underwater explosion, but on a very small scale. This sudden formation of bubbles cause mechanical stress on the cells which lead to cell destruction
1. Photodynamic Cancer Therapy:
Photodynamic therapy (PDT) is a treatment modality which involves administering a photosensitive agent and illuminating the target area to activate the agent. In cancer therapy, existing PDT agents react upon photostimulation to produce cytotoxic oxygen species, killing the target cell. Some of the advantages of PDT include its localized effects, reduced cost, and the fact that it is largely an outpatient therapy. Interestingly, it can also induce immune responses even against tumors that are not particularly immunogenic. One of its primary limitations, however, is the limited penetration depth of visible, infrared, and UV radiation into the patient and the lack of effective dosimetry techniques for PDT, making it difficult for dose distributions in the treatment volume to be accurately measured.
As of 2010, only three classes of photosensitisers for PDT had entered clinical use for cancer therapy, all of them chemical products rather than nanoparticles. However, amyriad of nanoparticle PDT agents are presented in various stages of development and show promise against a wide range of cancers. These nanoparticles can be separated into “passive” nanoparticles, which carry photosensitive agents, and “active” particles, which are they involved in the photostimulation process.
a) Passive Nanoparticles:A wide range of materials are in use for passive nanoparticles, including gold, polyacrylamide , silica, and biodegradable polymers. One of their primary advantages over unencapsulated photosensitizers is their ability to preferentially accumulate in target cells, which is of particular use given that most photosensitizers described so far in the literature have a low solubility in water and tend to accumulate in blood cells rather than tumors. This allows similar therapeutic effects to be achieved with lower doses of conventional photosensitizers such as hypericin, limiting their side effects. Encapsulating potentially toxic photosensitizers inside nanoparticles is another possibility, enabling their use in patients when this might not otherwise be possible.
One issue that must be considered in passive photodynamic nanoparticles is the optimum drug concentration within the nanoparticle to achieve maximum therapeutic effect, as excessive drug loading can reduce the overall effect of the nanoparticles.
There is increasing presence in the literature of biodegradable passive nanoparticles for photodynamic therapy, typically made from polylactic acid or poly (lactic-coglycolic acid). These nanoparticles can selectively deliver photosensitizers to target cells and then break down inside the cell, releasing the photosensitizer into target cells. This approach has seen impressive results in mouse models; in a study involving the administration of the photosensitizer hexadecafluoro zinc phthalocyanine to EMT-6 mammary tumors in mice, encapsulation of the photosensitizer in polylactic acid nanoparticles resulted in 100% of tested mice achieving tumor regression, compared with only 60% of mice administered the free drug. Nanoparticle encapsulation of Indocyanine green, recently evaluated as a possible photosensitizer, was observed to increase organ deposition to the photosensitizer to levels 2–8 times that for the free drug.
Other photosensitizers which have observed beneficial results from encapsulation in biodegradable nanoparticles include hypericin and zinc (II) phthalocyanine.
b) Active Nanoparticles: A rare example of nanoparticles that can act as photosensitizer on their own are titanium dioxide (TiO2) nanoparticles, which are receiving considerable attention as TiO2 is a known photocatalyst and reacts with water to produce oxidizing free radicals when exposed to UV light, which can result in localized damage to nearby cells. Recent in vitro studies on glioma cells have demonstrated the potential of such nanoparticles for photodynamic therapy. A similar effect has recently been produced in TiO2 nanoparticles with ultrasonic stimulation, which is able to kill nanoparticle-impregnated glioma cells when exposed to ultrasound in a similar manner to UVstimulated Nanoparticles. TiO2 nanoparticles are also essentially non toxic and hence show considerable promise as cancer therapy agents. Other nanoparticles that have recently been shown to demonstrate photosensitizer effects include porous silicon and carbon-60 buckyballs
Other active nanoparticles generally serve to enhance the photodynamic process and are still used in conjunction with a photosensitizer. The aim of these nanoparticles is usually to generate light at frequencies useful for photodynamic therapy in regions deeper inside the patient, sidestepping the issue of the limited penetration hampering photodynamic therapy at non superficial depths. The majority of studies in this area has focused on “upconverting” nanoparticles, which convert low-frequency near infrared radiation (NIR) into frequencies usable for existing photosensitizers. As well as penetrating further into the patient, NIR is also less damaging to the patient.
The most popular upconverting material for such nanoparticles at present is sodium yttrium fluoride doped with erbium and yttrium ions with a poly(ethylene imine) coat, forming PEI/NaYF4: Yb3+, Er3+ Nanoparticles.
Proof-of-concept studies in rats have confirmed that these upconverting nanoparticles can activate zinc phthalocyanine photosensitisers in rats, with resultant therapeutic effects, upon stimulation with NIR radiation well outside the absorption spectrum of the photosensitizer. Upconverting nanoparticles, their photosensitizer, and a biocompatible layer may be combined into “composite nanoparticles,” which can produce millimolar quantities of cytotoxic radicals upon illumination with NIR. The upconverting phosophor used in these nanoparticles may also be activated with X-ray radiation, which would completely eliminate the depth limitations of photodynamic therapy, at the expense of increased healthy tissue damage.
1. Photo thermal Therapy:
A diverse range of gold nanoparticles have been explored for use in therapy and imaging applications. Key features to consider when selecting a particle for hyperthermia are the wavelength of maximal absorption, the absorption cross-section, and the size of the particle. In this section, the development of several forms of gold-based nanoparticles used for therapy are discussed, including gold–silica nanoshells, gold nanorods, gold colloidal nanospheres, and smaller-diameter NIR-tunable gold nanocages, gold–gold sulfide nanoparticles, and hollow gold nanoshells.
i) Gold–Silica Nanoshells
ii) Gold Nanorods
iii) Small NIR-Tunable Gold Nanoparticles
iv) Gold Colloidal Nanospheres
v) Selecting a Therapeutic Gold Nanoparticle for Translation
i) Gold–Silica Nanoshells:
In 2003, Hirsch et al. were the first to demonstrate photothermal therapy using gold–silica nanoshells. Gold–silica nanoshells, composed of silica cores with a thin overlay of gold, were the first gold nanoparticles easily tunable to the NIR. By varying the size of the silica core and the thickness of the gold shell, the resonance of these nanoshells can span from the visible to the near infrared. Gold–silica nanoshell fabrication is based on seed-mediated growth, where ‘seeds’ of gold colloid are attached to the silica cores, and additional gold is added for completion of the shell. Similarly to other gold nanoparticles, gold– silica nanoshells have been studied specifically for their potential as imaging contrast agents with darkfield microscopy, two photon microscopy, reflectance confocal microscopy, and optical coherence tomography (OCT). In addition to having utility in cancer imaging, nanoshells that are strong absorbers can induce cancer cell death by converting light into heat (Figure2). Silica-based gold nanoshells have been tested in vitro as targeted-therapy probes for human breast, prostate, brain, and liver cancers. In addition, nanoshells have demonstrated in-vivo therapeutic efficacy against xenografted subcutaneous tumors in mice and allografted tumors in dogsThe larger size of gold–silica nanoshells as opposed to many other gold nanostructures provides an advantage in scatter based imaging, but in-vivo delivery may be more challenging than for smaller particles in some applications.
ii) Gold Nanorods:
Gold nanorods, which were developed during the same period as gold–silica nanoshells, are generally smaller than nanoshells. Like gold–silica nanoparticles, gold nanorods are easily tuned to the NIR region by simple manipulation of their aspect ratio (length/width) and have been extensively studied for cancer therapy applications. They have the advantages of small sizes (on the order of 10 nm × 50 nm, comparable to gold colloid particles), high absorption coefficients, and narrow spectral bandwidths. Owing to their distinctive rod shape, gold nanorods have two absorption peaks corresponding to the longitudinal and transverse resonances. The transverse resonance occurs at around 520 nm, while the longitudinal resonance can span the visible and NIR wavelengths. Recent studies have investigated the photothermal heating efficiencies of NIR-absorbing gold nanoparticles, and both theoretical and experimental results have shown that nanorods offer a superior absorption cross-section versus gold–silica and gold–gold sulfi de nanoparticles when normalizing for particle size differences, as well as heating per gram of gold that is at least six times faster than gold–silica nanoshells. However, gold–silica nanoshells have a significantly larger photothermal transduction cross-section when compared to gold nanorods on a per-particle basis. Nanorods have demonstrated success in vivo against oral squamous cell carcinoma and colon cancer xenografted into mice. Like gold–silica nanoshells, nanorods can be designed to function as imaging probes, and have been used to enhance the imaging contrast in darkfield microscopy and two-photon microscopy
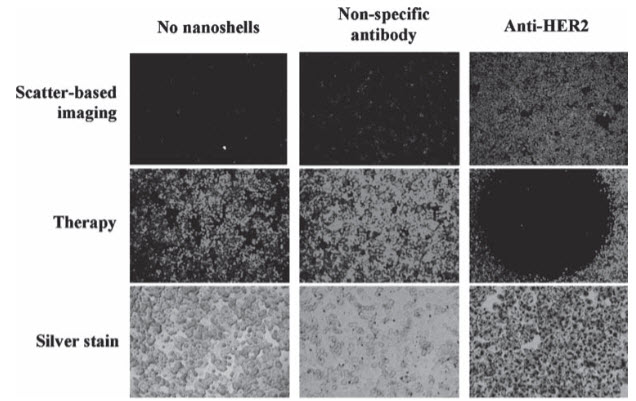
Figure 2. In-vitro imaging and therapy of human epidermal growth factor receptor 2 (HER2)-positive breast cancer cells using anti HER2-conjugated gold–silica nanoshells. The top row is darkfield microscopy of each treatment group, the middle row is a live stain of the cells after irradiation with NIR light, and the bottom row is a silver stain to show nanoshell binding. The same nanoshells are utilized to both image and ablate the breast cancer cells. Reproduced with permission
iii) Small NIR-Tunable Gold Nanoparticles:
The smaller size of gold–gold sulfide (GGS) nanoparticles, hollow gold nanoshells (HAuNS), and gold nanocages gives a clear delivery advantage for in-vivo photothermal ablation over larger gold nanoparticles. The first example of these smaller gold nanoparticles is gold–gold sulfi de nanoparticles, which are smaller than their gold silica counterparts with total diameters down to 25 nm. These particles are fabricated by the reduction of HAuCl 4 using sodium sulfide or sodium thiosulfate, and the synthesis produces two spectral peaks, one at approximately 520 nm due to gold colloid contamination, and one in the NIR region. After synthesis, extensive washing is required to remove contaminating gold colloid from the product.
These gold nanoparticles have an NIR absorbance that can be utilized for ablative therapy.
iv) Gold Colloidal Nanospheres
The final group of gold nanoparticles discussed for hyperthermia applications are gold colloidal nanospheres. Previously, these solid gold spheres were solely investigated for their use as imaging probes. However, the small size and relatively simple synthesis of these particles make them appealing for hyperthermia applications. A key disadvantage of these particles is their absorbance peak, which is in the visible region at around 530 nm. This wavelength is outside the NIR window, presenting a difficulty for in vivo studies.
Photothermal therapy with gold nanospheres has been explored in both the visible and near-infrared wavelength regions. the use of gold colloidal nanospheres for the imaging and therapy of oral cancer cells in vitro using a continuous argon laser at 514 nm, which is closely coincident with the peak absorbance of 40 nm particles. Compared to normal, noncancerous cells, it was demonstrated that cancerous cells targeted with nanoparticles were destroyed with 2–3 times lower laser power.
It uses a short-pulsed, NIR laser on small gold aggregates formed from 30 nm gold colloidal particles to selectively ablate oral cancer cells. By operating in the NIR, the laser power needed to kill the cancer cells was approximately 20 times less than that needed to destroy normal cells. Alternatively, a similar result has been achieved using gold nanoclusters and photothermal microbubbles (PTB)
Here, non-aggregated gold colloid is incubated with cancer cells and subsequently internalized via endocytosis. The nanoparticles are in close proximity to each other within the cell endosome, creating a shift in the nanoparticle absorbance to the NIR. Through the use of laser pulses in either the visible or NIR regions, vapor bubbles ranging from 10 − 8 to 10 − 4 m can be formed around the nanoclusters.
v) Selecting a Therapeutic Gold Nanoparticle for Translation:
The first decision is the selection of the gold nanoparticle variant. The efficiency with which a nanoparticle absorbs and scatters light is an essential parameter to consider when choosing a particle for use in an application. From a photothermal-therapy perspective, the absorptive cross section is an important variable. Calculating the absorption cross-section of gold-based nanoparticles can be achieved by using Mie-based theory or numerical methods such as a discrete dipole approximation. Particle design is optimized by maximizing the absorption efficiency at a desired wavelength in a specific surrounding medium in order to achieve a maximum particle temperature and surface heat flux.
The use of NIR light and nanoparticle absorbers in photothermal therapy offers critical advantages, particularly in protecting healthy tissue from thermal damage. However, solid tumors can occur within the body at depths greater than 1 cm, which is beyond the penetration of NIR light in tissue. In these cases, fiber-optic probes often can be used to deliver light.
ROLE IN CANCER DIAGNOSTICS –
- In cancer research, colloidal gold can be used to target tumors and provide detection using SERS (Surface Enhanced Raman Spectroscopy) in vivo. These gold nanoparticles are surrounded with Raman reporters that were stabilized when the nanoparticles were encapsulated with a thio-modifier –polyethylene glycol coat. To specifically target tumor cells the pegylated gold particles are conjugated with an antibody against Epidermal Growth Factor Receptor which is sometimes over expressed in cells of certain cancer types. Using SERS, these pegylated gold nanoparticles can then detect the location of the tumor.
- Gold nanorods are used for enhancing the overall signal, they are untargeted contrast enhancing agents which increase the endogenous vascular signal. They are important in many applications including cancer and drug delivery & are highly customizable for conjugating to molecules of interest or directing bioactivity.
- Chinese scientists have used gold nanoparticles as ultrasensitive fluorescent probes to detect cancer biomarkers in human blood.
- Nanodumbbells’ target cancer cells- US scientists have designed nanoparticles that function like ‘guided missiles’ in the targeted destruction of breast cancer. This approach could reduce side effects associated with anti-cancer drugs & has the potential to be adapted for different types of cancer, as well as wide range of other diseases.
- Rod-shaped gold particles attach to breast cancer proteins with specific antibodies, and a diagnosis can be made by examining a blood sample for its light-scattering characteristics.
Gold particles in cancer treatment:
Gold and Nanotechnology are in the age of innovation. Nanotechnology based cancer treatment approaches potentially provide localized targeted therapies that aim to enhance efficacy, reduce side effects and improve patient quality of life.
Kanzius RF Therapy- attaches microscopic nanoparticles to cancer cells and then “cooks” tumors inside the body with harmless radio waves. When the gold nanoparticles are inside the malignancy, a blast from a radio-frequency generator causes them to heat and cook the cancer cells.
Targeted nanoparticle delivery of therapeutic molecules has the potential to provide safer and more effective therapies for cancer applications. Scientists are developing a ‘golden-bullet against breast cancer’- heat tiny gold ‘nano-shells’ and injecting them directly into the breast cancer tissue.
References:
1. Wei Lu, Nanoparticles for Cancer Treatment, Medicine & Health/Rhode Islandm.
2. Mohini Sharma, Ashish Singh, Use of gold nanoparticles in Cancer Detection and Treatment, science daily.com/releases/2005/06/0506052434049.htm
3. Hirsch LR, Gobin AM, Lowery AR, Tam F, Drezek RA, Halas NJ, West JL. Metal nanoshells. Ann Biomed Eng. 2006; 34:15–22.
4. Schwartzberg AM, Olson TY, Talley CE, Zhang JZ. Synthesis, characterization, and tunable optical properties of hollow gold nanospheres. J Phys Chem B. 2006; 110:19935–44.
5. A. M. Gobin , M. H. Lee , N. J. Halas , W. D. James , R. A. Drezek , J. L. West , Nano Lett. 2007 , 7 , 1929
6. L. Bickford , J. Sun , K. Fu , N. Lewinski , V. Nammalvar , J. Chang , R. Drezek , Nanotechnology 2008 , 19 , 315102 .
7. Xiaohua Huang, Prashant K. Jain, Ivan H. El-Sayed & Mostafa A. El-Sayed, Plas- monic photothermal therapy (PPTT)using gold nanoparticles, Lasers Med Sci (2008) 23:217228
8. J. Park , A. Estrada , K. Sharp , K. Sang , J. A. Schwartz , D. K. Smith , C. Coleman , J. D. Payne , B. A. Korgel , A. K. Dunn , J. W. Tunnell , Opt. Express 2008 , 16 , 1590 .
9. M. Marsh, E. Schelew, S. Wolf & T. Skippon, Gold Nanoparticles for Cancer Treatment, March 29, 2009
10. Laura C. Kennedy , Lissett R. Bickford , Nastassja A. Lewinski , Andrew J. Coughlin ,Ying Hu , Emily S. Day , Jennifer L. West , * and Rebekah A. Drezek *, A New Era for Cancer Treatment: Gold-Nanoparticle- Mediated Thermal Therapies, wileyonlinelibrary.com, small 2010, X, No. XX, 1–15
11. Leon Smith,1 Zdenka Kuncic,1 Kostya (Ken) Ostrikov,1, 2 and Shailesh Kumar2, Nanoparticles in Cancer Imaging and Therapy, Journal of Nanomaterials Volume 2012, Article ID 891318, 7 pages
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









