{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Chauhan Sudhanshu*1,2, Savani Pankaj2, Solanki Divya2, Raj Hasumati2, Patel Sagar2
1Research Scholar 2015, Gujarat Technological University, Ahmedabad, Gujarat, India
2Department of Quality Assurance,
Shree Dhanvantary Pharmacy College, Kim, Surat, Gujarat, India
* sudhanshuchauhan32@yahoo.com
ABSTRACT
This review article presents the pharmacology of combined Candesartan Cilexetil and Pioglitazone Hydrochloride therapy specially in Metabolic syndrome. Candesartan Cilexetil is a antihypertensive agent. Pioglitazone Hydrochloride is a selectively stimulates nuclear receptor peroxisome proliferator activated receptor gamma (PPAR-gamma). The use of Candesartan Cilexetil in combination with Pioglitazone Hydrochloride has been proved to provide beneficial effect (Synergistic effect) in metabolic syndrome. The mechanism of Candesartan Cilexetil and Pioglitazone Hydrochloride is quite different. The combination of both also have anti inflammatory and enhanced organ protective effects. The main objective of this review article is to provide pharmacological information of combined therapy of Candesartan Cilexetil and Pioglitazone Hydrochloride to researcher in development of combined dosage form of this combination.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2413
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 6 Received On: 20/01/2016; Accepted On: 27/01/2016; Published On: 01/06/2016 How to cite this article: Chauhan S, Savani P, Solanki D, Raj H, Patel S; Review: Combined Candesartan Cilexetil and Pioglitazone Hydrochloride therapy in Metabolic Syndrome; PharmaTutor; 2016; 4(6); 23-28 |
INTRODUCTION

Figure 1: Diagrammatic representation of metabolic syndrome. [1]
I) Insulin resistance [2]
The most accepted hypothesis to describe the pathophysiology of the metabolic syndrome is insulin resistance. That is why the metabolic syndrome is also known as the insulin resistance syndrome. Insulin resistance has been defined as a defect in insulin action that results in hyperinsulinaemia, necessary to maintain euglycaemia. Concept of insulin resistance provides a conceptual framework with which to place a substantial number of apparently unrelated biological events into a pathophysiological construct. A major contributor to the development of insulin resistance is an overabundance of circulating fatty acids, released from an expanded adipose tissue mass. FFA reduce insulin sensitivity in muscle by inhibiting insulin-mediated glucose uptake. Increased level of circulating glucose increases pancreatic insulin secretion resulting in hyperinsulinemia. In the liver, FFA increase the production of glucose, triglycerides and secretion of very low density lipoproteins (VLDL). The consequence is the reduction in glucose transformation to glycogen and increased lipid accumulation in triglyceride (TG). Insulin is an important antilipolytic hormone. In the case of insulin resistance, the increased amount of lipolysis of stored triacylglycerol molecules in adipose tissue produces more fatty acids, which could further inhibit the antilipolytic effect of insulin, creating additional lipolysis.
II) Hypertension [2]
The relation between insulin resistance and hypertension is well established. Several different mechanisms are proposed. First, insulin is a vasodilator when given intravenously to people of normal weight, with secondary effects on sodium reabsorption in the kidney. In the setting of insulin resistance, the vasodilatory effect of insulin can be lost, but the renal effect on sodium reabsorption preserved. Fatty acids themselves can mediate relative vasoconstriction. Hyperinsulinaemia may result in increased sympathetic nervous system (SNS) activity and contribute to the development of hypertension.
III) Obesity and increased waist circumference [2]
Metabolic syndrome include abdominal obesity, pathogenesis of the metabolic syndrome and its components is complex, abdominal obesity is a key causative factor, metabolically obese, normal-weight individuals, having increased amount of visceral adipose tissue. According to some theories, with increases in visceral adipose tissue, a higher rate of flux of adipose tissue-derived free fatty acids to the liver through the splanchnic circulation would be expected, while increases in abdominal subcutaneous fat could release lipolysis products into the systemic circulation and avoid more direct effects on hepatic metabolism.
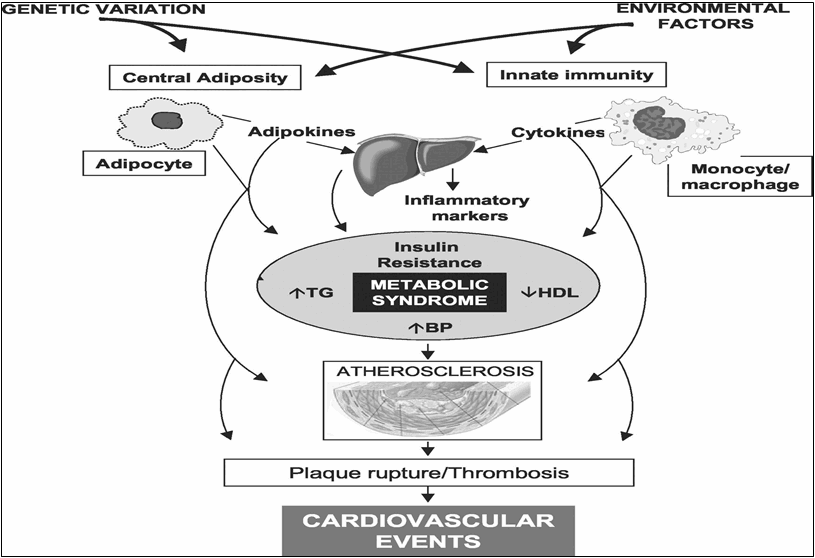
Figure 2: Pathophysiology of atherosclerotic cardiovascular disease in the metabolic syndrome.[3]
CANDESARTAN CILEXETIL
Chemical name:- (1RS)-1-(cyclohexyloxycarbonyloxy)ethyl-2-ethoxy-1-{[2’-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-benzo[d]imidazole-7-carboxylate. A Imidazole derivativeappearsas White crystals or white crystalline powder. [4] The drug is Practically insoluble in water and Sparingly soluble in methanol.Melting point about 163 ºC [5]. The pKa value is about 5.3 [6]

Figure 3: The chemical structure of Candesartan Cilexetil
PHARMACOKINETIC AND PHARMACODYNAMIC PROFILE [7]
Absorption
Candesartan cilexetil is a prodrug. It rapidly and completely bioactivated by ester hydrolysis during absorption from the gastrointestinal tract to candesartan. The absolute bioavaibility of Candesartan was estimated to be 15% after administration of the Candesartan cilexetil available as prodrug. Food with a high fat content has no affect on the bioavaibility of candesartan from candesartan cilexetil.
Distribution
The volume of distribution of candesartan is 0.13 L/kg. Candesartan is highly bound to plasma proteins about >99% and does not penetrate Red Blood Cells.
Metabolism and Excreation
Total plasma clearance of Candesartan is 0.37 ml/min/kg and renal clearance about 0.19 ml/min/kg. Candesartan when administered orally, about 26% of the dose is excreated unchanged in urine. For oral dose of 14C-labled Candesartan about 33% of radioactivity is recovered in urine and about 67% in feces. For Intravenous dose of it shows 59% of radioactivity is recovered in urine and about 36% in feces. Biliary system also contributes in elimination of candesartan.

Table I: Pharmacokinetics of Candesartan.
Pharmacodynamic
Candesartan inhibits the pressur effects of angiotensin II infusion in a dose-dependent manner. After 1 week of once-daily dosing with 8 mg of candesartan cilexetil, the effect of pressur was inhibited by approximately 90% at peak with approximately 50% inhibition persisting for 24 h. In a dose dependent manner after single and repeated administration of Candesartan cilexetil to healthy subjects and hypertensive patients increases plasma concentrations of angiotensin I and angiotensin II as well as plasma rennin activity. Plasma aldosterone concentrations did not influence by the once-daily administration of up to 16 mg of Candesartan cilexetil to healthy subjects, but when 32 mg of Candesartan cilexetil was administered to hypertensive patients it was observed that decrease in the plasma concentration of aldosterone.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PIOGLITAZONE HYDROCHLORIDE [4]
The chemical name is (5RS)-5-{4-[2-(5-Ethylpyridin-2-yl)ethoxy]benzyl}thiazolidine-2,4-dione monohydrochloride. Pioglitazone hydrochloride is a a white or almost White solid crystalline powder that is Practically insoluble in water, Soluble in methanol. Melting point about 193-194 ºC [5]. The pKa value is about 6.66
The structural formula is shown below:
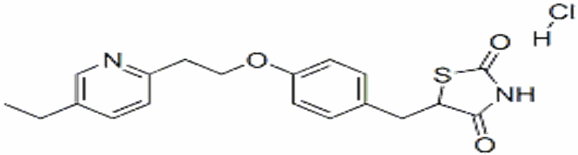
Figure 4: The structure of Pioglitazone Hydrochloride
PHARMACOKINETIC AND PHARMACODYNAMIC PROFILE: [8]
Pharmacokinetic:
Absorption
oral administration of pioglitazone in the fasting state, it is first measurable in serum within 30 minutes and with peak concentrations observed within 2 hours. Food slightly delays the time to peak serum concentration to 3 to 4 hours, but does not alter the extent of absorption.
Distribution
The mean apparent volume of distribution (Vd/F) of pioglitazone for single-dose administration is about 0.63 ± 0.41 (mean ± SD) L/kg of body weight. Pioglitazone is extensively protein bound (> 99%) in human serum, principally to serum albumin. It also bound to serum proteins but having a low affinity.
Metabolism
Pioglitazone is extensively metabolized by hydroxylation and oxidation. The metabolites also partly convert to glucuronide or sulfate conjugates. Metabolites M-II and M-IV (hydroxy derivatives of pioglitazone) and M-III (keto derivative of pioglitazone) are pharmacologically active in animal models of type 2 diabetes. In addition to pioglitazone, M-III and M-IV are the principal drug-related species found in human serum on multiple dosing. At steady-state in both healthy volunteers and in patients with type 2 diabetes, pioglitazone comprises about 30% to 50% of the total peak serum concentrations and 20% to 25% of the total AUC.
Excreation
On oral dose approximately 15% to 30% of the pioglitazone dose is recovered in the urine. Renal elimination of pioglitazone is negligible, and the drug is excreted primarily as metabolites and their conjugates. It is presumed that most of the oral dose is excreted into the bile either unchanged or as metabolites and eliminated in the faeces.
COMBINATION THERAPY OF CANDESARTAN CILEXETIL AND PIOGLITAZONE HYDROCHLORIDE
• Combination therapy improved BP/Glycemic control and was well tolerated. Thus, CC/PIO combination therapy is useful in patients with hypertension and type 2 diabetes mellitus. [9]
• Simultaneous Co-administration of Candesartan with Pioglitazone improved inflammatory parameters (HMW-ADN= High molecular adiponectin and PAI-1= Plasminogen activator inhibitor1), and improvements in inflammatory parameters were correlated with HbA1c. The co-administration of Candesartan with Pioglitazone could be an effective therapeutic strategy for treating hypertensive patients with Type 2 DM. Co-administration of candesartan with pioglitazone had beneficial effects with regard to hypertension, inflammation, and adipose tissue metabolism. [10]
• Metabolic Syndrome or a pre-diabetic condition, in a mammalian patient in need of such treatment, is disclosed comprising administering a dual PPARα/γ agonist and an Angiotensin II Type I receptor antagonist in an amount that is effective to treat hypertension and type 2 diabetes mellitus, Metabolic Syndrome or a pre-diabetic condition.
• The treatment of type 2 diabetes (non-insulin dependent diabetes mellitus, or type 2 diabetes mellitus) and to various disorders associated with type 2 diabetes mellitus treatment or amelioration of hypertension, hyperglycemia and hyperlipidemia associated with type 2 diabetes mellitus by administration of the combination of above active ingredients. Combination beneficial to the treatment of one or more other diseases or conditions that often accompany type 2 diabetes mellitus including lipid disorders, such as mixed or diabetic dyslipidemia, isolated hypercholesterolemia, elevated LDL-C and/or non-HDL-C, elevated hyperapo-B-liproteinemia, hypertriglyceridemia, elevated triglyceride-rich-lipoproteins, and low HDL cholesterol, by administration of the combination of active ingredients such as PPAR alpha-gamma Agonist like Pioglitazone Hydrochloride and Angiotensin-2 Antagonist like Candesartan Cilexetil or other Sartans.
• Treatment or amelioration of hyperglycemia, atherosclerosis and obesity by administration of the combination of active ingredients described above. The diseases listed above are treated or controlled by administration of a combination of a therapeutically effective amount of a PPARα/γ dual agonist and a therapeutically effective amount of an A-2 antagonist, including pharmaceutically acceptable salts of one or more of the active ingredients.
• The combination disclosed herein are useful in the treatment of type 2 diabetes, Metabolic Syndrome and associated disorders, and may be used as an adjunct to diet and exercise to decrease hypertension in patients who have type 2 diabetes or Metabolic Syndrome, and concurrently to control or ameliorate hyperglycemia and/or the dyslipidemia, hyperlipidemia, hypercholesterolemia and other lipid disorders that often occur in diabetic patients. In particular, the combination are effective for treating patients with type 2 diabetes with or Metabolic Syndrome.
• Furthermore, the combination are useful for reducing blood pressure, insulin resistance, hyperglycemia, elevated total-cholesterol, LDL-cholesterol, non-HDL-cholesterol, apolipoprotein B, and TG, and increasing serum HDL-C and Apolipoprotein A-I and A-II levels.
To summarize, the combination as defined above are useful in treating the hypertension associated with type 2 diabetes, pre-diabetic conditions, or Metabolic Syndrome, and for concurrently treating the following conditions that often accompany the above:
1. Lipid disorders
2. Hyperlipidemia
3. Obesity
4. Hypercholesterolemia
5. Hypertriglyceridemia
6. Dyslipidemia
7. Hyperglycemia
8. Insulin resistance
9. Low-HDL cholesterol
10. Atherosclerosis and sequallae of atherosclerosis, such as angina, claudication, heart attack and stroke.
• By treating, preventing, or minimizing the risk of developing the above disorders in a patient in need of such treatment, by administering to the patient a dual PPAR α/γ agonist in combination with an A-2 antagonist, said compounds being administered in an amount that is effective to treat, prevent, or minimize the risk of developing the above disorders.[11]
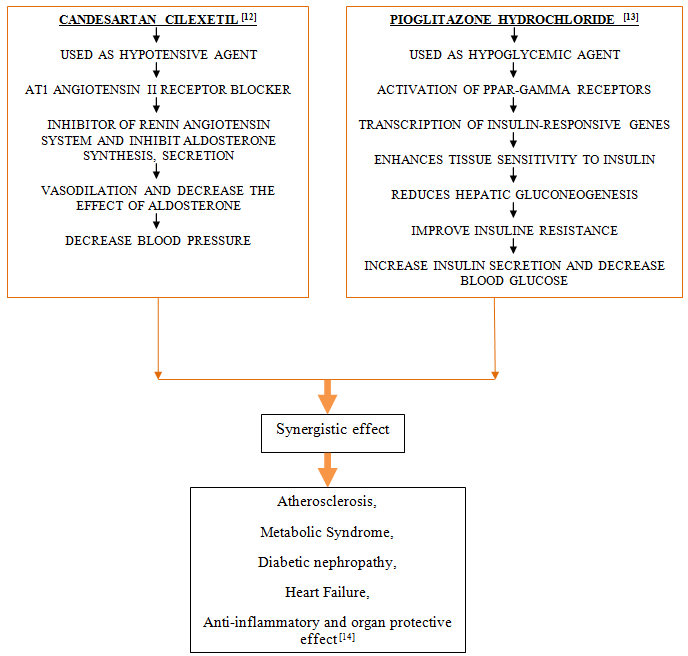
CONCLUSION
By reviewing the all literatures, the combination therapy was found to be effective in treatment of Metabolic syndrome, Diabetic nephropathy, Heart failure and beneficial as Anti-inflammatory and organ protective effect. This review represents individual pharmacology and pharmacokinetic of Candesartan Cilexetil and Pioglitazone Hydrochloride as well as mechanism of action of combination of Candesartan Cilexetil and Pioglitazone Hydrochloride in treatment. This review will helpful for researcher in future study and also for development of combined formulation.
REFERENCES
1. Kaur G., Mukundan S., Wani V. and Kumar M.S.; Nutraceuticals in the Management and Prevention of Metabolic Syndrome; Austin J Pharmacol Ther.; 2015; 3(1); 1063
2. Aganovic I. and Dusek T.; Pathophysiology of Metabolic Syndrome; The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine; 2007; 18(1); 1-5
3. Reilly M.P., Rader D.J.; The Metabolic Syndrome; Mini-Review: Expert Opinions; 2003; 1546-1551
4. Japanese Pharmacopeia, The Ministry of Health, Labour and Welfare, 16th Edition; Official From March 24; 2011; 511-1249
5. M.J O’Neil, P.E Heckelman, C.B Koch and K.J Roman; The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals; Fourteenth edition; Merck & Co.,Inc.; Whitehouse Station; NJ; USA; 2006; 291-1335
6. Gleiter C.H., Jagle C., Gresser U. and Morike K.; Candesartan; Cardiovascular Drug Reviews; 2004; 22(4); 263-268
7. Asif H., Sabir M.D., Mitra M. and Bhasin P.S., A review on candesartan: pharmacological and pharmaceutical profile; Journal of Applied Pharmaceutical Science; 2011; 1(10); 12-17
8. Radhika B., Vijayakumar S. and Ramaiyan D.; A pharmacokinetic interaction of pioglitazone and its clinical applications: A short review; International Journal of Pharmaceutical Sciences Letters; 2012; 2(1); 1-9
9. Kaku K., Enya K., Sugiura K. and Totsuka N.; Efficacy and Safety of combination therapy with Candesartan Cilexetil and Pioglitazone Hydrochloride in patients with hypertension and type 2 diabetes mellitus; Current Medicinal Research Opinion; 2011; 3; 73-84
10. Suzuki et al.; Effects of co-administration of Candesartan and Pioglitazone on inflammatory parameters in hypertensive patients with type 2 diabetes mellitus: a preliminary report; Cardiovascular diabetology; 2013; 12:71; 1-9
11. Jonathan C.F., Michele M. and Waldstreicher J.; Combination therapy using a dual PPAR alpha/gamma agonist and an Angiotensin ii type i receptor antagonist; United state patent; WO2004017896; 2004
12. Candesartan Drug Info in Drug bank. (Database Available On Internet), September 2015, drugbank.ca/drugs/DB00796
13. Pioglitazone Drug Info in Drug bank. (Database Available On Internet), September 2015, drugbank.ca/drugs/DB01132
14. Masae S., Yoshinori N., Muneo N. and Toshio Y.; Medicinal Composition; Japanese Patents; JP2009107944; 2009
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









