 About Author:
About Author:
Bandana patel
Shri Ram Institute Of Technology
Jabalpur(M.P.) 482002
Vandanapatel.mbbs@gmail.com
Abstract:
Tagetes erectus leaf were evaluated against various gram +ve and gram–ve bacteria and fungi, ethanol extracts exhibited very satisfactory inhibitory activity. Further studies involving the isolation, characterization and purification of the chemical compounds of the plant and screening for antibacterial and antifungal may result in the development of a potent entity which will be of lower toxicity and a high therapeutic value to the mankind.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1592
INTRODUCTION
Tagetes erecta, the Mexican marigold, also called Aztec marigold, is a species of the genus Tagetes native to Mexico and Central America. Despite its being native to the Americas, it is often called African marigold. In Mexico, this plant is found in the wild in the states of San Luis Potosí, Chiapas, State of México, Puebla, Sinaloa, Tlaxcala, and Veracruz. This plant reaches heights of between 50–100 cm (20–39 in). The Aztecs gathered the wild plant as well as cultivating it for medicinal, ceremonial and decorative purposes. It is widely cultivated commercially with many cultivars in use as ornamental plants.


[adsense:468x15:2204050025]
Physico chemical parameters:
The parameter was done to evaluate the percentage of total ash, water soluble acid insoluble ash were calculated as per Indian Pharmacopoeia. The extract of the powdered leaves were prepared with the different solvents for the study of extractive value. Fluorescence analysis was also carried out for the powder as well as different extracts.
Powder analysis: Preliminary analysis of the powder of leaf of Tagetes erectus were carried out with different chemical reagents.
Preliminary phytochemical analysis: For the Preliminary phytochemical analysis, the extract was prepared by weighing 100gm of dried powdered leaf and were subjected to maceration with different solvants as per the Polarity, Ethanol, Hydro-alcoholic, and finally with Aqueous. The extracts were filtered in each step, concentrated, and the solvent was removed by rotary evaporator. The extracts were dried over desiccators and the residues were weighed. The presence and absence of the primary and secondary phytoconstituents was detected by usual prescribed methods.
Plant material: The leaves of Tagetes erectus were collected from the wild sources of Shirpur forest, and it was identified and authenticated by Dr. Sagar Kshirsagar, Dept. of Botany, SSVPS, College of science, Dhulia. A voucher specimen is placed in the Dept. of Pharmacognosy for further reference. The collected plants were washed, dried and were pulverized with the help of mechanical grinder and was passed through sieve no 40, and stored in closed vessels for future use. The fresh leaves were used for Microscopy Identification.
Pharmacognostic Studies: Morphological Studies were carried out by using simple determination technique, the shape, size, color, odour, margin and apex. Apex of the leaf . Microscopic Studies were carried out by preparing of thin hand section of leaf. The sections were cleared with alcohol and stained as per the Protocol. Histochemical reaction were applied with Concentrated Hydrochloric Acid and Phloroglucinol and were mounted in Glycerin for identification of Lignified Elements, Iodine Solution for Identification of Starch Grains, 60% Sulphuric Acid for Calcium Oxalate Crystals in the powdered leaf by reported methods .
Macroscopic Characters of Leaf:
The transverse section of the leaf showed following characters. The leaf is generally dorsiventral in nature and it consisted of two major regions namely the Lamina region and Midrib. The lamina region consisting of upper and lower epidermis, spongy mesophyll region, which consisting of palisade cells and few crystals of calcium oxalate. The midrib region consisted byxylem and phloem. At the lower portion collenchyma cells which were completely arranged, above that loosely packed parenchyma cells were observed.
Powder Microcopy: The green colored powder was used for the study. The powder was stained with phloroglucinol and concentrated hydrochloric acid. It was mounted in glycerin and examined under 10 X and then magnified with 40 X. On microscopical examination it showed prism shaped calcium oxalate crystals. Trichomes were unicellular and uniseriate covring, vessels were spiral in nature, starch grains were spherical in nature, and fibres were long, slender and non-lignified in nature.
Fluorescence Analysis: The powder drug and extracts were subjected to fluorescence analysis as per the standard procedure.
Physicochemical Parameters: The powdered drug was evaluated for its physic-chemical parameters like Ash values: Acid Insoluble ash, water soluble ash, water insoluble ash, extractive values (Alcohol and water soluble values) and loss on drying.
Preliminary Phytochemical Analysis: The Alcoholic and Aqueous extract was subjected to preliminary phytochemical analysis for their presence of the constituents. It showed the presence of Flavonoids, tannins, phenols and glycosides –
Flavonoids are a group of polyphenolic compounds, which are widely distributed through out the plant kingdom. To date about 3000 varieties of flovonoids are known1. Many have low toxicity in mammals and some of them are widely used in medicine for maintenance of capillary integrity2. Flavonoids exhibit several biological effects such as antiinflammatory, anti
hepatotoxic and anti-ulcer actions3,4. They also inhibit enzymes such as aldose reductase and xanthine oxidase. They are potent antioxidants and have free radical scavenging abilities. Many have antiallergic, antiviral actions and some of them provide protection against cardiovascular mortality. They have been shown to inhibit the growth of various cancer cell lines in vitro, and reduce tumour development in experimental animals. In this review we have attempted to describe the present status of their classification, pharmacological/biochemical effects and their therapeutic potential.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Structure and classification of flavonoids:
Flavonoids occur as aglycones, glycosides and methylated derivatives. The flavonoid aglycone consists of a benzene ring (A) condensed with a sixmembered ring (C), which in the 2-position carries a phenyl ring (B) as a substituent (Figure 1). Six-member ring condensed with the benzene ring is either a pyrone (flavonols and flavonones) or its dihydroderivative (flavanols and flavanones). The position of the benzenoid substituent divides the flavonoid class into flavonoids (2-position) and isoflavonoids (3-position). Flavonols differ from flavonones by hydroxyl group the 3-position and a C2-C3 double bonds8. Flavonoids are often hydroxylated in position 3,5,7,2',3',4',5'. Methylethers and acetylesters of the alcohol group are known to occur in nature. When glycosides are formed, the glycosidic linkage is normally located in positions 3 or 7 and the carbohydrate can be L-rhamnose, D-glucose, glucor-hamnose, galactose or arabinose9. I. Pharmacological Effects of Flavonoids.
1). Antimicrobial activity:
Flavonoids and esters of phenolic acids were investigated for their antibacterial, antifungal and antiviral activities. All samples were active against the fungal and gram-positive bacterial test strains and most showed antiviral activity.
2) Antifungal Activity: Number of flavonoids isolated from peel of tangerine orange, when tested for fungistatic activity towards Deuterophoma tracheiphila showed promising activity. Chlorflavonin was the first chlorine-containing flavonoid type antifungal antibiotic produced by strains of Aspergillus candidus.
PHYTOCHEMICAL SCREENING
Test for flavonoids :
Two grams of the ethanolic extract was weighed, placed in a test tube, followed by the addition of 10 ml of DMSO. The mixture was heated, followed by the addition of magnesium metal and 6 drops of concentrated hydrochloric acid. The appearance of red colour was indicative of the presence of flavonoids. Same procedure was repeated using aqueous extract (f) Test for alkaloids One gram each of the ethanolic extract was weighed and placed into two separate test tubes. To the first test tube, 2-3 drops of Dragendoff’s reagent was added while 2-3 drops of Meyer’s reagent were added to the second test tube. The development of an orange-red precipitate (turbidity) in the first test tube (with Dragendoff’s reagent) or white precipitate (turbidity) in the second test tube (with Meyer’s reagent) was indicative of the presence of alkaloids. Same procedure was repeated using aqueous extract (Cuilel, 1994).
Test for saponins :
Five grams of the aqueous extract was weighed and placed in a test tube. This was followed by the addition of 5 ml de-ionised distilled water. The content was vigorously shaken. The appearance of a persistent froth that lasted for 15 minutes was indicative of the presence of saponins. Same procedure was repeated using DMSO for the ethanolic extract (Brain and Turner, 1975).
Test for tannins :
Two grams of the aqueous extract was weighed and placed in a test tube. Two drops of 5% ferric chloride solution was then added. The appearance of a darkgreen color was indicative of the presence of tannins. The same procedure was repeated using the ethanolic extract (Cuilel, 1994).
Test for steroid glycosides :
One gram of the ethanolic extract was weighed and placed in a test tube. This was dissolved in 2 ml of acetic anhydride, followed by the addition of 4 drops of chloroform. Two drops of concentrated sulphuric acid were then added by means of a pipette at the side of the test tube. The development of a brownish ring at the interface of the two liquids and the appearance of violet colour in the supernatant layer were indicative of the presence of steroid glycosides. Same procedure was repeated using the aqueous extract (Cuilel, 1994).
Extraction and Isolation of Thiophenes from Tagetes
The air-dried roots of Tagetes (500 g) were chopped and soaked in ethanol for 15 days. This procedure was repeated thrice. The combined extract evaporated under vacuum at 40°C and then the gummy residue was suspended in water, then partitioned first with hexane and this hexane extract was subjected to column chromatography. Two compounds were eluted with hexane: chloroform (9:1), 5′-hydroxymethyl-5-(3-butene-1-ynyl)-2,2′-bithiophene (Heywood V.H. and Harborne J.B. ed., 1977) and another compound eluted in n-hexane:chloroform (8:2), 5′-methyl-5-[4-(3-methyl-1-oxobutoxy)-1-butynyl]-2,2′-bithiophene (The Wealth of India, 1976).
Extraction and Isolation of Steroids and Terpenes
The air-dried flowers (1300 g) were soaked in ethanol. Kept at room temperature for 15 days, and this procedure was repeated thrice. The combined ethanolic extract was evaporated under reduced pressure. The ethanolic gummy residue (64.4 g), so obtained, was suspended in water, then partitioned first with hexane, and thereafter with ethylacetate in order to remove neutral substances and other impurities. The aqueous portion was extracted several times with presaturated n-BuOH. The n-BuOH fractions were pooled together and evaporated under high vacuum. Each extract, i.e. n-hexane, ethyl acetate extract and n-BuOH extract were examined by thin layer chromatography (TLC). Preliminary chemical screening (chromogenic reactions on TLC plates) of hexane extracts with ceric ammonium sulphate showed sterols constituents and thiophenes and flavonoids. The n-hexane fraction of flower was subjected to column chromatography on silica gel using a mixture of solvent systems; which afforded four compounds i.e. β-sitosterol in n-hexane-ethyl acetate (9:1); stigmasterol in n-hexane:ethylacetate (7:3) whereas some semipure fractions obtained by the same column, which were mixed together and further subjected to column chromatography and isolated cholesterol and lupeol in n-hexane: ethylacetate (6:4), nhexane: ethylacetate (5:5) respectively.
Collaction of Tegetes erecta:
Irrespective of the type of crude drug and area of collection, there cannot be 2 opinion that the drugs are collected suitably when they contain maximum concentration of active constituents. the advantages of existing environmental condition is also taken into consideration while collection of crude drug. Marigold is generally found near gardens as well as dry areas.
Harvesting: . leaves are collected from plant.
Drying: Drying consists of removal of excess moisture content of crude drug, so as to improve its quality and make it resistant to microbial contamination. Hence the collected plants were dried for sufficient amount of time under shade to prevent loss of volatile constituents.
METHODOLOGY
ANTIMICROBIAL ACTIVITY OF PLANT EXTRACT OF TAGETES ERECTA
A BRIEF INTRODUCTION:
Drug substances that either suppress or influence the growth of micro organisms are generally analysed by microbial method. Two procedures are generally employed in microbial assay. The increased prevalence of antibiotic-resistant bacteria due to the extensive use of antibiotics may render the current antimicrobial agents inefficient to control some bacterial diseases (Tanaka et al., 2006). Herbal medicine is frequently a part of a larger therapeutic system such as traditional and folk medicine. It is necessary to evaluate, in a scientific base, the potential use of folk medicine for the treatment of infectious diseases produced by common pathogens. Medicinal plants might represent an alternative treatment in non-severe cases of infectious diseases. They can also be a possible source for new potent antibiotics to which pathogen strains are not resistant. The search and use of drugs and dietary supplements derived from plants have been accelerated in recent years. Ethnopharmacologist, botanist, microbiologist and natural product chemist are combing the medicinal flora for biological substances that could be developed for the treatment of infectious diseases. Several medicinal plants have been extensively studied in order to find more effective and less toxic compounds. Pure extract of an herb’s ‘active component’ are more reliable and safer than administration of the herb itself. Many herbs are now in use whose therapeutic properties and active principle are as yet not well understood. Plant derived natural products such as flavonoids, terpenoids, and steroids have received considerable attention in recent years due to their diverse pharmacological properties including antioxidant and antitumor activity.
Two procedures are generally employed in microbial assay
1.CYLINDER PLATE METHOD(CUP):
This is based on measurement of the diameter of microbial growth inhibition surrounding the cylinders containing various dilutions of the test compound which are placed on the surface of a solid nutrient medium previously inoculated with a culture of suitable microbe. Inhibition produced by the test compound is compared with that produced by known concentration of a refrence standard.
TURBIDIMETRIC METHOD:
Based on inhibition of microbial growth as in indicated by measurement of turbidity (transmittance) of suspension of a suitable micro-organism in a fluid medium, to which have been added graded amounts of the test compounds. Changes in the transmittance produced by the tested compounds are compared with those produced by known concentration of standard.
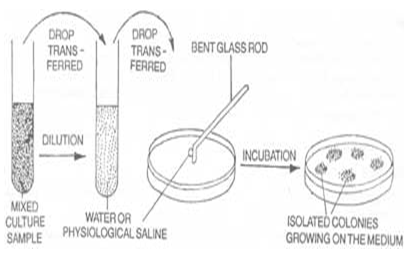
Material and Methods
Method of extraction:-
The fresh plant parts were collected, properly washed in tap water, rinsed in sterile distilled water and then air dried in the hot air oven to remove moisture and to dry them. They were then grounded soxhlet extracted using 70% ethanol. The extraction lasted for 24 hours.
Antimicrobial susceptibility testing: -
The antimicrobial potential of the above plant extracts was seen against the test organisms using the agar-gel diffusion susceptibility test. Sterile Mueller – Hinton plates were taken one plate/organism tested. Three wells of about 3.0 mm diameter were aseptically punched on each agar plate using a sterile cork borer, with at least 30 mm distance between adjacent wells and the peripherary. According to the standard technique of Opara and Anasa (1993) - 2-4 colonies of the test organisms were inoculated in sterile broth and these inoculums was swabbed using sterile swab on the surface of above punched
Mueller - Hinton agar plates. A fixed volume (0.1 ml) of theplant extract was then introduced into the wells in the increasingconcentration and then incubated at 370C for 24 hours. Theresulting zones of inhibition were measured.
Requirements:
Aquous as well as ethanolic extract of eclipta alba, B.subtilis, E.coli.nutrient agar medium, cup & plate.
PROCEDURE:
1. Two bacteria, (gram +ve i.e.B.subtilis, gram-ve i.e E. Coli) were used in the present study to determine the antibacterial activity of the crude extracts by agar diffusion method (cup plate method).
2. In the agar diffusion method, nutrient agar for bacteria used as culture media and cavity were aseptically made over the culture platesusing borer (9mm internal diameter).
3. The cavities were filled with extracts, standards and control The plates were incubated at 37 0 C for 24 hrs.
4. The activities were determined by measuring the diameter of the zone in mm.
5. The experiment was replicated two times to confirm the reproducible results.
6. Solvent used as negative control in each time. Standard Ciprofloxacin (100mcg/0.1ml), Amoxicillin (100mcg/0.1ml) for bacteria were used as positive control for comparison of the activities.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Observation table
|
S.No |
EXTRACT OF MADAR LEAVES |
ZONE OF INHIBITION in E.coli |
ZONE OF INHIBITIONINBACILLUS SUBTILUS |
|
3 |
Ethanolic extract |
28.3mm |
22.6mm |
|
4 |
Aquous extract |
23.4mm |
20.4mm |
|
Treatment |
Conc. mg/ml |
E. coli Zone of inhibition |
B. subtilis Zoneof inhibition |
|
Chloroform extract |
50 100 |
7 8 |
8 10 |
|
Ethanolic extract |
50 100 |
09 14 |
09 13 |
|
Water extract |
50 100 |
08 10 |
09 10 |
RESULT
The results of these investigation showed that plant extracts of tagetes erecta possess appreciable and potential antimicrobial activity against commonly encountered microorganisms in humans. It is interesting to note that the action of the extracts of it is non toxic Studies.
ANTIFUNGLE ACTIVITY OF TAGETUS ERECTA
A BRIEF INTRODUCTION:
Drug substances that either suppress or influence the growth of fungus are generally analysed by fungle method .Two procedures are generally employed in microbial assay.The increased prevalence of antibiotic-resistant bacteria due to the extensive use of antibiotics may render the current antimicrobial agents inefficient to control some bacterial diseases (Tanaka et al., 2006). Herbal medicine is frequently a part of a larger therapeutic system such as traditional and folk medicine. It is necessary to evaluate, in a scientific base, the potential use of folk medicine for the treatment of infectious diseases produced by common pathogens. Medicinal plants might represent an alternative treatment in non-severe cases of infectious diseases. They can also be a possible source for new potent antibiotics to which pathogen strains are not resistant. The search and use of drugs and dietary supplements derived from plants have been accelerated in recent years. Ethnopharmacologist, botanist, microbiologist and natural product chemist are combing the medicinal flora for biological substances that could be developed for the treatment of infectious diseases. Several medicinal plants have been extensively studied in order to find more effective and less toxic compounds. Pure extract of an herb’s ‘active component’ are more reliable and safer than administration of the herb itself. Many herbs are now in use whose therapeutic properties and active principle are as yet not well understood. Plant derived natural products such as flavonoids, terpenoids, and steroids have received considerable attention in recent years due to their diverse pharmacological properties including antioxidant and antitumor activity.
Two procedures are generally employed in fungle assay
1.CYLINDER PLATE METHOD(CUP):
This is based on measurement of the diameter of microbial growth inhibition surrounding the cylinders containing various dilutions of the test compound which are placed on the surface of a solid nutrient medium previously inoculated with a culture of suitable microbe.Inhibition produced by the test compound is compared with that produced by known concentration of a refrence standard.
TURBIDIMETRIC METHOD:
Based on inhibition of microbial growth as in indicated by measurement of turbidity(transmittance)of suspension of a suitable micro-organism in a fluid medium,to which have been added graded amounts of the test compounds.Changes in the transmittance produced by the tested compounds are compared with those produced by known concentration of standard.
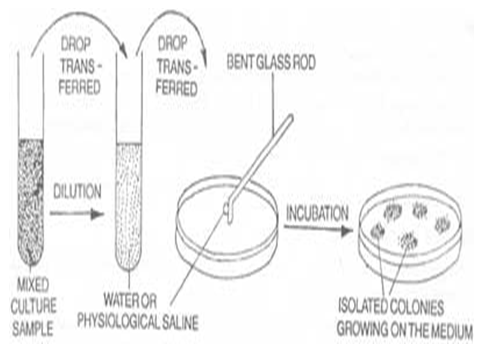
Material and Methods
Preparation of Various Extracts
Air-dried powdered leaves (1 kg) were exhaustively extracted by Soxhlet’s apparatus successively by increasing order of polarity with petroleum ether, chloroform, ethyl acetate and ethanol. The aqueous extract was prepared by cold maceration of 250 g of the shade dried leaf powder in 500ml of chloroform water (1:99) for 7 days. The various extracts obtained were filtered, concentrated, dried in vacuum and the residue stored in a refrigerator at 2-8° C for use in subsequent experiments.
Requirements:
Fungus Aspergillus niger and Candida Albicans. Aquous as well as ethanolic extract of eclipta alba ,.nutrient agar medium, cup & plate.
PROCEDURE:
1. Fungus Aspergillus niger and Candidaalbicans.were use in the present study to determine the antibacterial activity of the crude extracts by agar diffusion method (cup plate method).
2.In the agar diffusion method, nutrient agar for fungus used as culture media and cavity were aseptically made over the culture platesusing borer (9mm internal diameter).
3.The cavities were filled with extracts, standards and control The plates were incubated at 37 0 C for 24 hrs.
4.The activities were determined by measuring the diameter of the zone in mm.
5.The experiment was replicated two times to confirm the reproducible results.
Observation table
|
Treatment |
Conc. mg/ml |
Zone of Inhibition (in mm) C. albicans |
Zone of Inhibition (in mm) A. niger |
|
Chloroform extract |
50 100 |
10 11 |
11 13 |
|
Ethanolic extract |
50 100 |
09 13 |
10 16 |
|
Water extract |
50 100 |
08 09 |
07 11 |
RESULT
the ethanolic extract of Tagetes erectus as most active against, E.coli in the dilution of 100 mg/ml. The chloroform extracts showed less activity than ethanol extract, but showed more activity than water extracts. Table 2 revealed that the ethanolic and chloroform extracts are more active against C. albicans and A. niger, whereas No activity was found in aqueous extract.
CONCLUSION
It can be concluded that while screening of various extracts of Tagetes erectus leaf against various gram +ve and gram–ve bacteria and fungi, ethanol extracts exhibited very satisfactory inhibitory activity. Further studies involving the isolation, characterization and purification of the chemical compounds of the plant and screening for antibacterial and antifungal may result in the development of a potent entity which will be of lower toxicity and a high therapeutic value to the mankind. These activities may be due to the presence of phytoconstituent present in the extract and the exact constituent responsible for the activity can be confirmed with the help of isolation techniques.
REFRENCE:
1. The Wealth of India (Anonymous), C.S.I.R. publication, New Delhi, India, 2005, p. 109
2. Khare C.P. Encylopedia of Indian Medicinal Plants, Springer-Verlag Berlin Heidelberg, New York, 2004, p. 441.
3. KKokate C.K. Practical Pharmacognosy, Vallabh Prakashan, New Delhi, 1994, p. 107.
4. Harborne J.B. Phytochemical Methods, Chapman and Hall, London, 1998, p.60
5. Paech K. Tracey M.V. (Eds.) Modern Methdender Pflanzenanalyse, Vol. III. Springer Verlag, Berlin, 1955, p.626.
6. Spooner, DF., and Sykes, G., (1972). Methods in Microbiology, Vol. VII B, Academic Press, London and New York, p. 216.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









