About Author
*Anzar Alam, Sanjay Kumar, H S Tiwari
Multani Pharmaceuticals Limited T-10,
Okhla Industrial Area Phase-II, New Delhi
ABSTRACT
For the grant of licence to manufacture Ayurvedic drugs for sale or distribution, an application in form 24-D is made to Licensing Authority appointed by state government in this behalf. A licence is issued for manufacture in 25-D subject to the fulfilment licensing conditions such as compliance to GMP (Schedule T), competent technical persons, documented records and requirements with respect to products. The requirement for safety and effectiveness evidences for issuance of product licence depends upon product type i.e. classical or propriety, ingredient such as presence or absence of Schedule E(1) ingredient, indication i.e. new or as per text, solvent for extraction i.e. aqueous or hydro-alcoholic etc.
INTRODUCTION
Ayurveda is the sacred system of health care originated in India. It is gaining widespread acceptability due to more affordable, more closely corresponds to the patient’s ideology, and less paternalistic than allopathic medicine. Earlier the practicing physicians used to prepare drug products by themselves for their patients. Today drug products are usually prepared and packed by manufacturer and made available to pharmacy or physician or health care systems for sale or distribution. Drug and Cosmetic Act 1940 (DCA) and Rules 1945 (DCR) have regulations for the manufacture of Ayurvedic drugs for sale or distribution. In addition to DCA, it is mandatory to comply all relevant laws such as Factory Act, Pollution Acts, GST registration etc. while manufacturing Ayurvedic drug for sale or distribution.
Factory means any premises engaged in “manufacturing process” employing 10 or more workers with the aid of power or, 20 or more workers without the aid of power. A person who runs a “factory” either in owned or rented premises shall be the “Occupier” and requires to obtain “factory” licence. State government may make rules regarding approval, licencing and registration of factories. The occupier of the factory is required to submit the application for the registration and grant of licence to state Chief Inspector (or Concern Inspector) along with required fee and documents such as ID proof of Occupier and Manager, list of Partners/Directors with their residential address, NOC from other partners or Board Resolution by Directors for nomination of occupier as per sections 2(n) and 7 of the Factories Act, 1948, proof/supporting documents of Occupier as Director/ Partner/ Proprietor of the factory, existing building plan, latest electricity bill as a proof of sanctioned load of electricity, proof of occupancy, flow chart of manufacturing process, list of raw materials used in manufacturing process, list of machineries installed in the premises etc. The application is scrutinized, followed by communication to applicant and inspection of premises. If factory conforms to the statutory requirements, the factory licence is granted for a period of one or five or ten years as per the application from the date of grant of licence. A licence granted under the provisions of Factories Act and Rules is required to be renewed for continuation of manufacturing activities. Licencing, registration process and requirement vary for state to state and place to place 1, 8, 9.
A 100 per cent pollution-free environment is a myth with operating factories and industries. Adequate and effective pollution control measures are required so that adverse effects on the environment are minimised. The three main laws relating to pollution are- The Water (Prevention and Control of Pollution) Act- 1974, The Air (Prevention and Control of Pollution) Act- 1981 and The Environment (Protection) Act-1986. There are numerous rules under these acts relating to different matters prohibiting industries from spread of pollution. As per the Water (Prevention and Control of Pollution) Act- 1974 and the Air (Prevention and Control of Pollution) Act- 1981, it is mandatory to obtain Consent to Establish (CTE) from respective state pollution control board, prior to commencement of construction or any similar activities to start the business. The process for obtaining CTE involves making an application in a prescribed format to respective pollution control board along with required documents and fee. It is followed by physical inspection and assessment of the environment management system proposed so as to meet the requirement prescribed. Once the industry or process plant is established along the required pollution control systems, the entrepreneur is required to obtain consent to operate (CTO) the unit. This consent is given for a particular period, which needs to be renewed regularly. In the present study an attempt has been made to understand the requirement for manufacture of Ayurvedic drugs at factory premises with reference to DCA 10, 11, 12, 13.
APPLICATION FOR LICENCE TO MANUFACTURE AYURVEDIC DRUGS
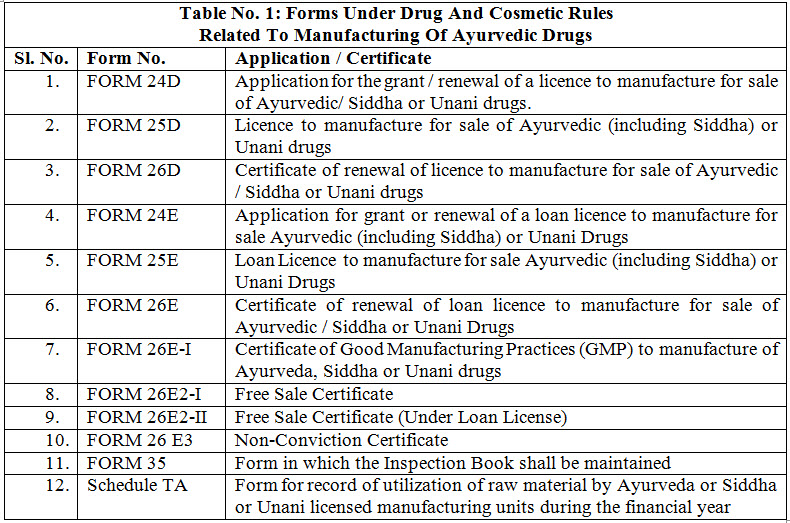
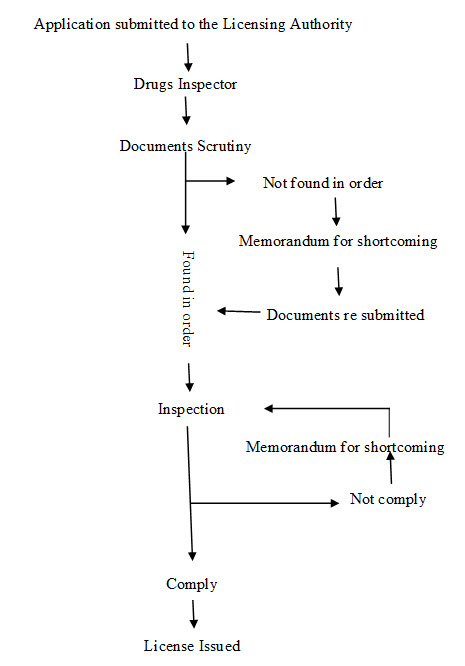
Manufacture of Ayurvedic drugs for sale or for distribution are permitted under the licence issued in Form 25-D by Licencing Authority (LA) appointed by state government in this behalf. The application for the grant or renewal of a licence is made in Form 24-D along with fee and documents such as declaration, site plan, key plan, ownership deed of the land, non-conviction affidavit, organization chart, authorized signatory, list of machineries, list of books, SOPs, proof of the premises, MOA, documents regarding medicines etc. which vary state to state or area to area to the LA appointed by state government. The LA consult the experts appointed in this behalf and grant licence only when the conditions of licencing are fulfilled for a period of five years from the date of issuance of the license. Fig. 1 shows the steps for licensing of Ayurvedic firms. For renewal of a licence, application is made to LA in Form 24-D and the certificate of renewable is issued from LA in Form 26-D. The conditions which are to be fulfilled for licencing include- GMP, competent technical staff and maintain of records. LA verify the requirements as per schedule T and issue the Good Manufacturing Practices (GMP) certificate in form 26 E-I, simultaneously along with grant or renewal of licence in form 25-D. There is also the provision for loan licence. A loan licence means a licence which a LA may issue to an applicant who does not have his own arrangements for manufacture but intends to avail himself of the manufacturing facilities owned by a licence in Form 25-D. Table 1 enlist Forms No. related with Ayurveda manufacturing units under DCR. These forms are applicable for Siddha and Unani for same purpose 3, 14, 15.
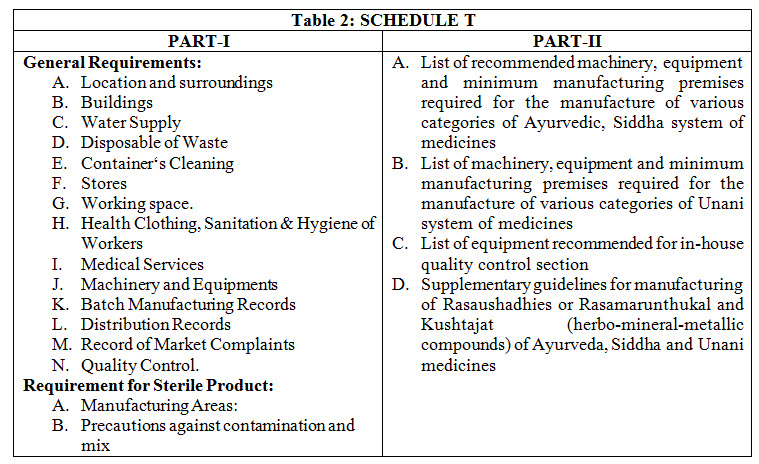
GOOD MANUFACTURING PRACTICE (GMP): GMP is the system for ensuring manufactured products are free from unexpected contamination, complies quality standards, safe for use and have expected therapeutic efficacy. GMP covers all aspects of production; from the starting materials, premises and equipment to the training and personal hygiene of staff. There must be system to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process- each time a product is made. DCR require the compliance to GMP for grant or renewal of license to manufacture any Ayurveda drug. Schedule “T” of DCR prescribes the GMP guidelines. Part-I of the schedule prescribes general requirement of GMP whereas part-II enlist recommended machinery and space for manufacturing and quality control (Table 2). There is supplementary guidelines in part-II for manufacturing of mineral/metal based formulations in Schedule “T”.7
The factory building shall be so situated and shall have such construction as to avoid all types of contamination. It should have proper exits and fire safety measures. The premises used for manufacturing, processing, packaging and labelling will be in conformity with the provisions of the Factory Act. The building design and construction, sanitary fitting and electrical fixtures, water supply and drainage system, drying space, furnace section and all other areas and parts of factory shall be such as to permit production of drugs under hygienic condition. Separate provision shall be made for lavatories to be used by men and women, and such lavatories shall be located at places separated from the processing rooms. A minimum of 1200 square feet covered area with separate cabins or partitions for each activity is required for Ayurveda manufacturing unit. Each manufacturing unit will have a separate area (minimum of 200 sq. feet) for Bhatti, furnace boilers, puta, etc. This will have proper ventilation, removal of smoke, prevention of flies, insets, dust etc. The furnace section could have tin roof. The minimum recommended machine and space for manufacturing of different Ayurvedic dosage are as in Table 3. One machine indicated for one category of medicine could be used for the manufacturing of other category of medicine also. Store should provide independent adequate space for storage of raw material (RM), packaging material (PM) and finished goods (FG). Store of RM may be sub-divided to handle different categories of raw materials. Appropriate containers which would protect the quality of RM in all aspect should be used with label indicating name of material, name of supplier, batch no, date of receipt and also the status of under test/approved/rejected. All the packaging materials, containers and closure, bottles, jar etc. shall be stored properly. The manufacturing area shall provide adequate space for orderly placement of equipment and material to facilitate easy and safe working and to minimize or to eliminate any risk of mix-up. The equipment/machines have to be properly installed and maintained with proper standard operational procedures (SOPs) for cleaning, maintaining and performance.
All workers employed in the factory shall be free from contagious diseases and there should be adequate facilities for first aid and medical examination of workers at the time of employment and periodical check-up once a year. Adequate facilities for personal cleanliness such as clean towels, soap and scrubbing brushes shall be provided. The uniform shall include cloth or synthetic covering for hands, feet and head wherever required. Workers will also be provided facilities for changing their clothes and to keep their personal belongings. Batch manufacturing record (BMR) of medicines shall provide an account of the list of RM and their quantities obtained from the store, tests conducted during the various stages of manufacture, in process record of various shodhana, bhavana, burning in fire, specific grindings, record of date, manpower, machine and equipment used, details of transfer of manufactured drug to the finished products store including dates, quantity, packaging, testing report of the FG and any other records as required. These records shall be duly signed by production and quality control personnel respectively. The FG transferred from the production area shall be stored in the FG stores within an area marked “Quarantine”. After the quality control have approved the correctness of FG in all aspect then it will be moved to “Approved Finished Goods Stock” area and goods shall be dispatched as per marketing requirements from this area only with records of sale and distribution of each batch. The record shall be maintained up to the date of expiry of the batch. A register shall be maintained to record all reports of market complaints and shall enter all data received on such market complaints, investigations carried out by the manufacturers regarding the complaint as well as any corrective action initiated to prevent recurrence of such market complaints shall also be recorded7.
|
Table 3: List Of Recommended Machinery, Equipment And Minimum Manufacturing Premises Required For The Manufacture Of Various Categories Of Ayurvedic Medicines |
|||
|
Sl. No. |
Category of Medicine |
Space required |
Machinery/ Equipment Recommended |
|
1 |
Anjana/Pisti |
100 sq. feet |
Mechanized / Motorized Karel, End Runner / Ball – mill, Sieves/ Shifter. |
|
2 |
Churna / Nasya / Manjan / Lepa / Kwath Churn |
200 sq. feet |
Grinder / Disintegrator / Pulveriser, Powder mixer, Sieves / Shifter. |
|
3 |
Pills / Vati / Gutika / Matirai and Tablets |
100 sq. feet |
Ball Mill, Mass mixer / Powder Mixer, Granulator, Drier, Tablet Compressing Machine, Pill / Vati Cutting Machine, Stainless Steel Trays / Container for storage., Sugar coating pan & Polishing pan in case of sugar-coated tablets, Mechanised chattoo for mixing guggulu where required. |
|
4 |
Kupi Pakava/Ksara/ Parpati/ Lavana Bhasma Satva / Sindura Karpu/Uppu / Param |
150 sq. feet |
Bhatti, Karahi/Stainless steel Vessels/Patila, Flask, Multani Matti / Plaster of Paris, Copper Rod, Earthen container, Gaj Put Bhatti, Mufflefurnace (Electrically operated), End/Edge Runner, Exhaust Fan, Wooden / S.S.Spatula. |
|
5 |
Kajal |
100 sq. feet |
Earthen lamps for collection of Kajal, Triple Roller Mill, End Runner, Sieves, S.S.Patila, Filling/ packing and manufacturing room should be provided with exhaust fan and ultra violet lamps |
|
6 |
Capsules |
100 sq. feet |
Air Conditioner, De-humidifier, Hygrometer, Thermometer, Capsule filling machine, Chemical balance. |
|
7 |
Ointment/Marham Pasai |
100 sq. feet |
Tube filling machine, Crimping Machine, Ointment Mixer, End Runner/ Mill (Where required), S.S. Storage Container S.S.Patila. |
|
8 |
Pak/Avaleh/Khand/ Modak/Lakayam |
100 sq. feet |
Exhaust fan fitted and fly proof Bhatti section, Iron Kadahi /S.S. Patila, S.S. Storage container |
|
9 |
Panak, Syrup / Pravahi Kwath Manapaku |
150 sq. feet |
Tincture press, Exhaust fan fitted and fly proof Bhatti section, Bottle washing machine, Filter press / Gravity filter, Liquid filling machine, P.P. Capping Machine. |
|
10 |
Asava / Arishta |
200 sq. feet |
Same as mentioned above., Fermentation tanks, Distillation plant and containers where necessary, Filter Press |
|
11 |
Sura |
100 sq. feet |
Same as mentioned above, Distillation plant, Transfer pump. |
|
12 |
Ark / Tinir |
100 sq. feet |
Maceration tank, Distillation plant, Liquid filling tank with tap, Gravity filter/Filter press, Visual inspection box. |
|
13 |
Tail/Ghrit/Ney |
100 sq. feet |
Bhatti, Kadahi / S.S. Patila, S.S.Storage containers, Filtration equipment, filling tank with tap, Liquid filling machine. |
|
14 |
Aschyotan / Netra Malham Panir/Karn Bindu/Nasa- bindu |
100 sq. feet |
Hot air oven electrically heated with thermostatic control, Kettle gas or electrically heated with suitable mixing arrangements, Collation mill, or ointment mill, Tube filling |
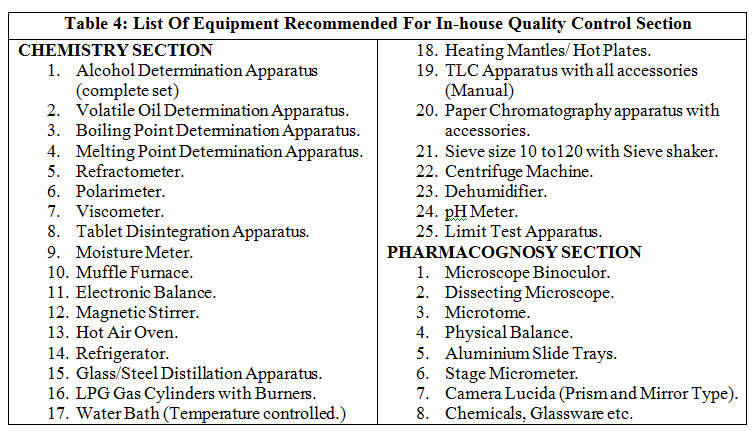
Every licensee is required to provide facility for quality control (QC) section in his own premises or through government approved testing laboratory. The test shall be as per the Ayurvedic pharmacopoeia standard and where the tests are not available, the test should be performed according to the in-house specification or other information available. Preferably for quality control section, there will be a separate expert. QC section requires150 sq. feet area, reference books and reference samples for identification of raw drugs, controlled samples of finished products of each batch will be kept till the expiry date of product. Manufacturing record should be maintained for the various processes.
Table 4 enlist equipment recommended for in-house QC.
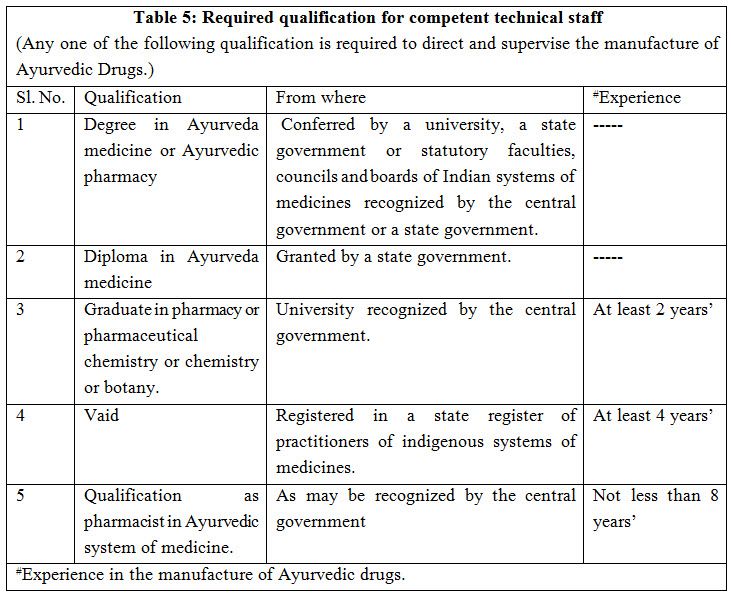
COMPETENT TECHNICAL STAFF: The manufacture of Ayurvedic drugs shall be conducted under the direction and supervision of competent technical staff consisting at least of one person, who is a whole time employee and who possesses the qualifications as shown in Table-5. Documents required for approval of competent staff include- degree, registration, biodata, experience, affidavit, photographs, appointment letter and consent letter etc. which vary state to state or area to area3.
RECORDS AND INSPECTION: The manufacturing unit shall keep a record of all RM utilized in the manufacture of Ayurvedic drugs in the preceding financial year, and shall submit the same by the 30th day of June of the succeeding financial year in proforma given in Schedule TA to the LA and to the National Medicinal Plants Board (NMPB) or any agency nominated by the NMPB for this purpose. The licensee shall maintain proper records of the details of manufacture and of the tests of RM and FG. The licensee shall allow an inspector appointed under the Act to enter any premises, to inspect the premises, to take samples of RM as well as FG, and to inspect the records maintained under these rules. The licensee shall maintain an inspection book in Form 35 to enable an inspector to record his impressions and the defects noticed3,
GUIDELINES FOR PRODUCT PERMISSION (License With Respect To Products): As defined in clause (a) of section 3 of DCA, “Ayurvedic drug includes all medicines intended for internal or external use for or in the diagnosis, treatment, mitigation or prevention of disease or disorder in human beings or animals, and manufactured exclusively in accordance with the formulae described in, the authoritative books”. More than 54 books are listed as authoritative books for Ayurveda in the first schedule of DCA. Ayurvedic drug defined above are of textual reference and in day-to-day language they are called classical medicine. Use of any prefix or suffix with the name of any classical formulation is not permitted except as described in the authoritative books. A formulation without any specific name, described in the authoritative books may be named on the basis of the ingredients of the formulation. Sub-clause (i) of clause (h) of section 3 of DCA defines patent and proprietary drug in relation to Ayurveda as “all formulations containing only such ingredients mentioned in the formulae described in the authoritative books of Ayurveda systems of medicine specified in the First Schedule, but does not include a medicine which is administered by parenteral route and also a formulation included in the authoritative books”. The name of any Ayurvedic falling under classical medicine shall not be used for naming any patent or proprietary medicine relating to Ayurvedic except for single plant-ingredient based Ayurvedic formulation licensed or to be licensed as patent or proprietary medicine. In DCR, patent or proprietary medicines have been categories under four broader categories. The newly added categories are- Balya/Poshak, Saundarya Prasadak and Aushadh Ghana. Formulations having ingredients mentioned in books of first schedule of the DCA and recommended for promotional and preventive health called Balya/Poshak/ Positive health Promoter whereas formulation having ingredients mentioned in books of First Schedule of DCA and recommended for oral, skin, hair and body care are Saundarya Prasadak.
Aushadh Ghana (Medicinal plant extracts - dry/wet) are extract obtained from plant mentioned in books of first schedule including aqueous or hydro-alcohol. In addition to efficacy, safety is the prime requirement for medicinal product. Schedule E(1) separately lists poisonous substances under Ayurvedic, Siddha and Unani system of medicine. If ingredient of Schedule E(1) is present in medicinal product for internal use of human then their container must be labelled conspicuously with the word ‘Caution: To be taken under medical supervision’ both in English and Hindi language. Evidence-based studies are becoming essential for establishing the safety and efficacy of drug products. DCA has given to attention toward safety and efficacy of drug products while issuing product permission. For issue of licence to the medicine with respect to Ayurvedic drug, the conditions relating to safety study and the experience or evidence of effectiveness shall be such as specified Table 6. Documents required for licensing the product include- affidavit, formulation, label, trial report, testing protocol, proof and product proforma etc. which vary state to state or area to area2, 3, 5, 6.
|
Table 6: Safety Study And Effectiveness Evidence For Issuance Of Licence For Ayurvedic Drugs |
||||||
|
Sl. No. |
Category |
Ingredients |
Indications |
Safety |
Experience/Evidence of Effectiveness |
|
|
Published Literature |
Proof of effectiveness |
|||||
|
Classical Ayurvedic drugs |
||||||
|
1 |
-as such |
As per text |
As per text |
Not required |
Required |
Not required |
|
2 |
-any change in dosage form. |
As per text |
As per text |
Not required |
Required |
Not required |
|
3 |
- to be used for new indication |
As per text |
New |
Not required |
If required |
Required |
|
Patent or Proprietary Ayurvedic drugs |
||||||
|
4 |
-with no ingredients of Schedule E(1) of D & C Act |
As per text |
Textual Rationale |
Not Required |
Of ingredient |
Pilot study as per relevant protocol for Ayurveda |
|
5 |
- with any of the ingredients of Schedule E(1) of D & C Act
|
As per text |
Existing |
Required |
Required |
Required |
|
Aushadh Ghana extract of medicinal plant (dry/wet) |
||||||
|
6 |
Aqueous extract |
As per text |
As per text |
Not required |
Required |
Not required |
|
7 |
Aqueous extract for new indication. |
As per text |
New indication |
Not required |
Required |
Required |
|
8 |
Hydro-alcoholic extract. |
As per text |
As per text |
Not required |
If required |
Not required |
|
9 |
Hydro-alcoholic for new indication |
As per text |
New indication |
Required |
If required. |
Not required |
|
10 |
Other than Hydro/ hydro-alcoholic extract.
|
As per text |
As per text |
Required Acute, Chronic, Mutagenicity and teratogenicity. |
Required |
Required |
For issue of licence with respect to Balya and Poshak medicines the person who applied for licence is required to submit the- (i) photo-copy of the textual reference of ingredients used in the formulation as mentioned in the book of 1st schedule; (ii) conduct safety studies in case the product contains of any of the ingredients as specified in the Schedule E (1), as per the guidelines for evaluation of Ayurveda Siddha and Unani Drugs formulations; (iii) for textual indications the safety and effectiveness study is not required. Same are the requirement with respect to Saundarya Prasadak. For licencing of proprietary medicine scientific data based shelf life or date of expiry based on real time stabilities of medicine in accordance with the guidelines prescribed in the Ayurvedic Pharmacopoeia of India, Part-I, volume-viii is also required to be submitted. In an order Ministry of AYUSH has ordered to all State Licencing Authority to consider and accept the accelerated stability data for fixing the shelf life for grant of license and renewal of licence3, 4, .
Conclusion:
A licence issued in Form 25-D by Licencing Authority is required to manufacture any Ayurvedic drug for sale or distribution. The conditions of licencing are GMP, competent technical staff, documented information and records, quality control through in-house or government approved lab, safety study and/or proof of effectiveness for product permission. The manufacturer has to comply other regulatory requirement related with factory, environment and business.
References:
1. Anonymous. The Factory Act 1948. In : Taxmann’s Factory Act 1948, Delhi, Taxmann Publications (P.) Ltd., 2016.
2. Anonymous. Chapter I, The Drug & Cosmetics Act 1940. In : Commercial’s Manual on Drug & Cosmetics, 10th ed., Garg RA. editor. Delhi, Commercial Law Publishers (India) Pvt. Ltd., 2018, 1-6.
3. Anonymous. Part XVI Manufacture for Sale of Ayurvedic (including Siddha) or Unani Drugs, The Drug & Cosmetics Rues 1945. In : Commercial’s Manual on Drug & Cosmetics, 10th ed., Garg RA. editor. Delhi, Commercial Law Publishers (India) Pvt. Ltd., 2018, pp 194-203.
4. Anonymous. Part XVII Labelling, Packing and Limit of Alcohol in AAyurvedic (Including Siddha) or Unani Medicine, The Drug & Cosmetics Rues 1945. In : Commercial’s Manual on Drug & Cosmetics, 10th ed., Garg RA. editor. Delhi, Commercial Law Publishers (India) Pvt. Ltd., 2018, pp 208-211.
5. Anonymous. Schedule E(1), The Drug & Cosmetics Rule 1945. In : Commercial’s Manual on Drug & Cosmetics, 10th ed., Garg RA. editor. Delhi, Commercial Law Publishers (India) Pvt. Ltd., 2018, 321.
6. Anonymous. Schedule I, The Drug & Cosmetics Act 1940. In : Commercial’s Manual on Drug & Cosmetics, 10th ed., Garg RA. editor. Delhi, Commercial Law Publishers (India) Pvt. Ltd., 2018, 54-57.
7. Anonymous. Schedule T, The Drug & Cosmetics Rues 1945. In : Commercial’s Manual on Drug & Cosmetics, 10th ed., Garg RA. editor. Delhi, Commercial Law Publishers (India) Pvt. Ltd., 2018, 574-589.
8. Procedure For Online Registration And Grant Of Licence Under The Factories Act, 1948 https://labour.delhi.gov.in/sites/default/files/All-PDF/FAQ_Factory.pdf cited on January 14th, 2021 07:33.
9. Procedure For Online Renewal Of Licence Under The Factories Act, 1948 https://labour.delhi.gov.in/sites/default/files/All-PDF/Renewal_factory_faq..pdf cited on January 14th, 2021 07:38.
10. The Water (Prevention And Control Of Pollution) Act, 1974 https://www.indiacode.nic.in/bitstream/123456789/1612/1/a1974-06.pdf cited on January 14th, 2021 07:17.
11. The Air (Prevention And Control Of Pollution) Act, 1981 https://www.indiacode.nic.in/bitstream/123456789/1389/1/a1981-14.pdf#search=The%20Air%20(Prevention%20and%20Control%20of%20Pollution)%20Act-%201981 cited on January 14th, 2021 07:47.
12.The Environment (Protection) Act, 1986 https://www.indiacode.nic.in/bitstream/123456789/1876/1/a1986-29.pdf#search=The%20Environment%20(Protection)%20Act-1986 cited on January 14th, 2021 07:52.
13. Procedure for obtaining consent to operate under Water Act, 1974 & Air Act, 1981 and authorization under Hazardous & Other Waste (M&TM) Rules, 2016. https://hspcb.gov.in/CTO_PRO.pdf cited on January 14th, 2021 08:06.
14.http://health.delhigovt.nic.in/wps/wcm/connect/50d2d2004e823985a88bfb2eb287f0d8/Steps+for+Licencing+of+Ayurvedic+and+Unani+Firms.pdf?MOD=AJPERES&lmod=-1636038194&CACHEID=50d2d2004e823985a88bfb2eb287f0d8 cited on January 20th, 2021 15:23
15.http://health.delhigovt.nic.in/wps/wcm/connect/e1c4e8804e823cb8a89dfb2eb287f0d8/Check+List+for+Licence+on+25D.pdf?MOD=AJPERES&lmod=-1624695340&CACHEID=e1c4e8804e823cb8a89dfb2eb287f0d8 cited on January 20th, 2021 15:20
DOWNLOAD THIS ARTICLE AS PDF >>
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









