ABOUT AUTHORS:
S.P.Sethy*, Tahseen Sameena, Prathima Patil, K.Shailaja
Department Of Pharmaceutical Chemistry.
Sushrut Institute of Pharmacy
Taddanpally (V), Pulkal (M), Medak-502293
sarada9439504350@gmail.com
ABSTRACT:
The complexity of today’s pharmaceutical market requires more efficient drug development and production. Product Lifecycle Management (PLM) has the opportunity to make pharmaceutical production more effective and with lower risk – even in this vastly complex environment. The product lifecycle management creates and manages a company's product-related intellectual capital starting from an idea to its final retreat. In pharmaceutical industry, it benefits through enhancing the lifespan of patent and pricing strategies. Improved patient compliance, revenue growth, expanded clinical benefits; cost advantages life extension exclusivity and quicker market launch are amongst the main applications of product lifecycle management. Leaders are actively implementing PLM and are reaping the benefits of fewer problems, lower costs, higher yields, employees armed to make good decisions, and audits that make everyone more confident as they access the information they need. The present manuscript focuses on product lifecycle management, problems and the key solutions for a successful product lifecycle management in pharmaceuticals.
REFERENCE ID: PHARMATUTOR-ART-2051
INTRODUCTION:
Product lifecycle management(PLM) is the process of managing the entire lifecycle of a product from its conception, through design and manufacture, to service and disposal. PLM forms the product information backbone for a company and its extended enterprise. It is composed of multiple elements including: foundation technologies and standards (e.g., XML, visualization, collaboration, enterprise application integration, etc.), information authoring and analysis tools (e.g., mechanical design, electronics design, software engineering, technical publishing, finite element analysis, etc.), core functions (e.g., data vaults, document and content management, workflow, product structuring, program management, etc.), functional applications (e.g., configuration management, engineering change control, etc.), and business solutions (e.g., new product introduction, supply chain collaboration, etc.) that incorporate best practices and methods.
In recent years the pharmaceutical industry has faced declining R&D productivity,a rapidly changing healthcare landscape and fierce competition from generics resulting in lower growth and profit margins. Historically, drug development focused on clinical trials management and outcomes. Now however, the industry is looking at more holistic approaches to improve processes of bring new products to market that can accelerate product development while lowering operational costs. This is challenging because of the complex value chain and business processes required in this highly regulated environment. Additionally, it has proven difficult for the industry to effectively adapt as many pharmaceutical companies are simply not optimized for cross functional collaboration which is so desperately needed to support these changing market conditions. One meaningful and holistic approach to today’s current challenges within the pharmaceutical industry is to focus on Product Lifecycle Management (PLM), which is a business transformation approach to manage products and related information across the enterprise. In recent years PLM has provided many pharmaceutical organizations with the ability to increase their ability to get products to market quicker, ensure greater regulatory compliance and efficiencies while reducing development costs. This article identifies some key business metrics that benchmark a company’s performance and key strategic business processes required to improve R&D performance through a PLM business transformation approach.
PHASES OF PRODUCT LIFECYCLE AND RELATED TECHNOLOGIES:
PHASE 1: CONCEIVE [IMAGINE, SPECIFY, PLAN, INNOVATE]
The first stage is the definition of the product requirements based on customer, company, market and regulatory bodies’ viewpoints. From this specification, the product's major technical parameters can be defined. In parallel, the initial concept design work is performed defining the aesthetics of the product together with its main functional aspects. Many different media are used for these processes, from pencil and paper to clay models to 3D CAID (computer-aided industrial design software). In some concepts, the investment of resources into research or analysis-of-options may be included in the conception phase – e.g. bringing the technology to a level of maturity sufficient to move to the next phase. However, life-cycle engineering is iterative. It is always possible that something doesn't work well in any phase enough to back up into a prior phase – perhaps all the way back to conception or research. There are many examples to draw from.
PHASE 2: DESIGN [DESCRIBE, DEFINE, DEVELOP, TEST, ANALYZE AND VALIDATE]
This is where the detailed design and development of the products form starts, progressing to prototype testing, through pilot release to full product launch. It can also involve redesign and ramp for improvement to existing products as well as planned obsolescence. The main tool used for design and development is CAD. This can be simple 2D drawing / drafting or 3D parametric feature based solid/surface modeling. Such software includes technology such as Hybrid Modeling, Reverse Engineering, KBE (knowledge-based engineering), NDT (Nondestructive testing), and Assembly construction. This step covers many engineering disciplines including: mechanical, electrical, electronic, software (embedded), and domain-specific, such as architectural, aerospace, automotive ... Along with the actual creation of geometry there is the analysis of the components and product assemblies. Simulation, validation and optimization tasks are carried out using CAE (computer-aided engineering) software either integrated in the CAD package or stand-alone. These are used to perform tasks such as:- Stress analysis, FEA (finite element analysis); kinematics; computational fluid dynamics(CFD); and mechanical event simulation (MES). CAQ (computer-aided quality) is used for tasks such as Dimensional tolerance (engineering) analysis. Another task performed at this stage is the sourcing of bought out components, possibly with the aid of procurement systems.
PHASE 3: REALIZE [MANUFACTURE, MAKE, BUILD, PROCURE, PRODUCE, SELL AND DELIVER]
Once the design of the product’s components is complete the method of manufacturing is defined. This includes CAD tasks such as tool design; creation of CNC Machining instructions for the product’s parts as well as tools to manufacture those parts, using integrated or separate CAM computer-aided manufacturing software. This will also involve analysis tools for process simulation for operations such as casting, molding, and die press forming. Once the manufacturing method has been identified CPM comes into play. This involves CAPE (computer-aided production engineering) or CAP/CAPP – (production planning) tools for carrying out factory, plant and facility layout and production simulation. For example: press-line simulation; and industrial ergonomics; as well as tool selection management. Once components are manufactured their geometrical form and size can be checked against the original CAD data with the use of computer-aided inspection equipment and software. Parallel to the engineering tasks, sales product configuration and marketing documentation work take place. This could include transferring engineering data (geometry and part list data) to a web based sales configuration and other desktop publishing systems.
PHASE 4: SERVICE [USE, OPERATE, MAINTAIN, SUPPORT, SUSTAIN, PHASE-OUT, RETIRE, RECYCLE AND DISPOSAL]
The final phase of the lifecycle involves managing of in service information. Providing customers and service engineers with support information for repair and maintenance, as well as waste management/recycling information. This involves using tools such as Maintenance, Repair and Operations Management (MRO) software. There is an end-of-life to every product. Whether it be disposal or destruction of material objects or information, this needs to be considered since it may not be free from ramifications.
PRODUCT AND PROCESS LIFECYCLE MANAGEMENT (PPLM):
Product and process lifecycle management (PPLM) is an alternate genre of PLM in which the process by which the product is made is just as important as the product itself. Typically, this is the life sciences and advanced specialty chemicals markets. The process behind the manufacture of a given compound is a key element of the regulatory filing for a new drug application. As such, PPLM seeks to manage information around the development of the process in a similar fashion that baseline PLM talks about managing information around development of the product. One variant of PPLM implementations are Process Development Execution Systems(PDES). They typically implement the whole development cycle of high-tech manufacturing technology developments, from initial conception, through development and into manufacture. PDES integrate people with different backgrounds from potentially different legal entities, data, information and knowledge and business processes.
PRODUCT LIFE CYCLE MANAGEMENT IN PHARACEUTICAL:
Management of the LAB to LAUNCH Process:
The Pharmaceuticals Industry faces three key challenges today:
1. Complex Drug Development Process.
2. Large Gaps between R&D Operational Performance and Strategic Importance.
3. Difficulty in managing Clinical Trial Inventories.
COMPLEX DRUG DEVELOPMENT PROCESS:
The drug development process is complex, consisting of many interrelated business activities and functional constituents participating in the “Lab to Launch” of any given product (Fig-1)
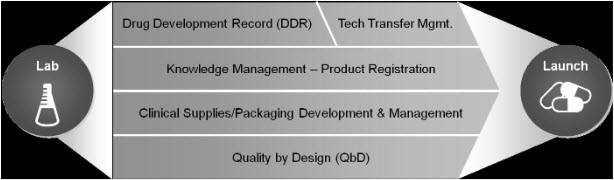
Figure 1 “Lab to Launch” Process
Real-time synchronization of these activities is critical for achieving improved performance and regulatory compliance in the R&D pipeline. Effective management, knowledge re-uses and accurate monitoring across these core activities requires automation. Automating will also enable standardization based on best practices and consolidation of content across the R&D pipeline, forming a compliant dataset for Quality by Design (QbD) based submissions.
LARGE GAP BETWEEN R&D OPERATIONAL PERFORMANCE AND STRATEGIC IMPORTANCE:
To address the development process, the pharmaceutical industry has identified key R&D functions that are considered important in optimizing R&D pipeline effectiveness (AMR). This research indicates significant gaps exist between R&D operational performance and strategic importance resulting in the industry operating at less than 50% effectiveness (Fig-2).And how a poor capital allocation lead to decline in pharmaceutical R& D Innovation (fig-3)
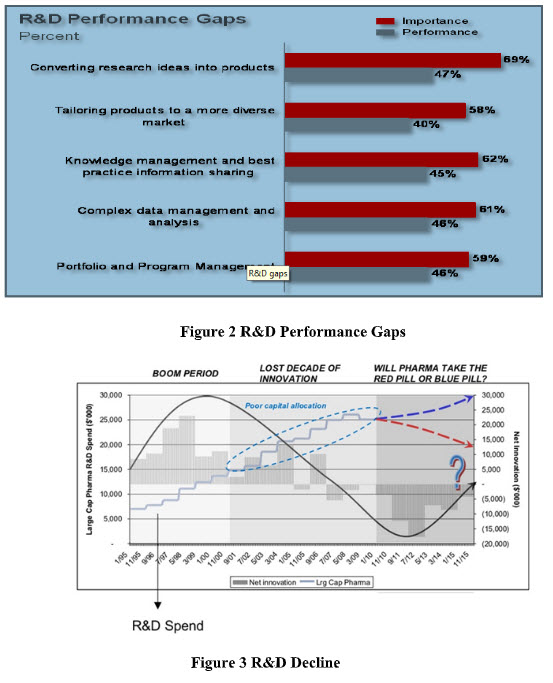
Figure 2 R&D Performance Gaps; Figure 3 R&D Decline
Finally, some of the key influencers that have commonly impacted profitability, risk and growth can be traced back to three fundamental issues within the industry itself
Increasing internal and external complexity in managing the entire product lifecycle from product inception to phase out due to the simple fact that many pharmaceutical organizations suffer from silos of information across the different functional areas. In the case of R&D organizations this is typically based on therapeutic areas whereby cross-functional information flow is either lacking or non-existent.
No single data source for products and related information due to a variety of different data sources and lack of collaboration across the organization. This often results in disparate, redundant and in worst cases inaccurate product information depending on functional area.
Gap Between R&D and Commercialization: Historically R&D processes have been largely viewed as independent of product launch and subsequent commercialization efforts within the industry thus resulting in a fundamental gap for coordinated and transparent collaboration.
HARD TO MANAGE CLINICAL TRIAL INVENTORIES:
A critical element of the drug development process is the production and management of the clinical trial inventory. Effective management of the “chain of custody” of this initial clinical inventory is difficult and becomes more complex as Contract Manufacturing Organizations (CMOs) and other external partners are utilized in this strategic process. Traditional inventory automation tools such as ERP or MES systems do not provide the flexibility needed at this stage of R&D production. Instead manual disjointed processes supported with desktop tools such as Excel® are often utilized resulting in unnecessary process and coordination complexity. Lack of precise coordination of the clinical trial inventory within the trial management plan is disruptive, adding considerable cost and time to this phase of product development (AMR). Consequently, key clinical supplies metrics routinely result in less than 25% of their targeted performance objectives (Figure 4).
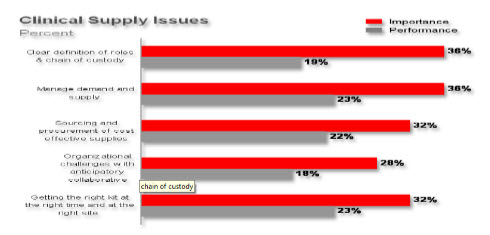
Figure 4 Clinical Supplies “Chain of Custody”
This combination of poor execution of the R&D pipeline and compromised production efficiency of the initial clinical supply process results in inadequate R&D results (AMR). Industry metrics based on project timeline performance, project cost, expected financial margin, and market share capture, show that approximately only 1 in 3 programs achieve their expected performance targets (Figure 4).
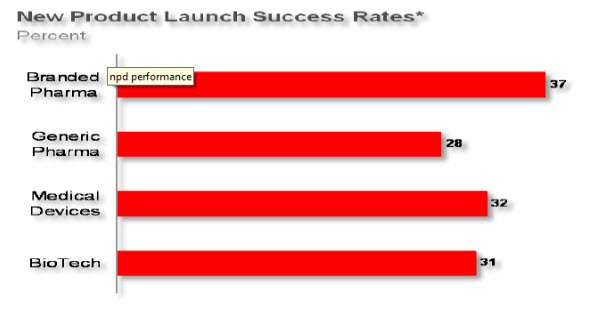
Figure 5 Current R&D Pipeline Performances
TRANSFORMING THE PHARMACEUTICAL INDUSTRY: Accept the major changes that happening in the pharmaceutical market space and understanding what the change is.

AN OPPORTUNITY TO IMPROVE R&D: While there are significant challenges within the pharmaceutical industry, opportunities exist in this increasingly competitive landscape for innovative companies looking for ways to transform their business that lead to profitability and growth. Companies that successfully manage the transformation process to address these challenges will realize improved business performance and differentiation in the market place as a result. As companies look to speed up the process by which new products are brought through the development pipeline to commercialization while supporting new therapeutic areas, a business transformation focused on cross functional collaboration whereby product knowledge can be uniformly leveraged will result in both productivity and revenue gains. It is also important to appreciate that even small incremental improvements can produce significant results in both revenue growth and margin (Oracle customer business cases). For example, for every day a company can reduce from the overall development cycle, they can realize significant reductions in cost and provide significant returns in both profitability and margins (Figure 6)
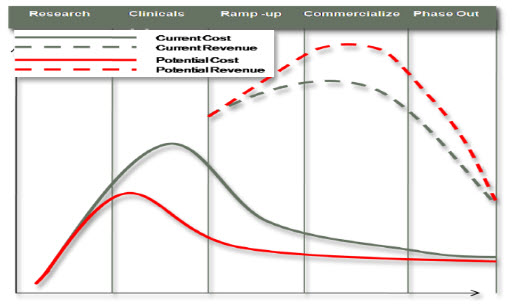
Figure 6 R&D Pipeline Potential Improvements
ACHIEVING R&D IMPROVEMENT: To achieve this, companies should apply the mantra of “think big - start small - scale fast” for any initiative related to improving development and manufacturing of clinical supplies. This will allow the enterprise to prioritize on a few key initiatives, standardize on those processes, and expand through a process of continuous improvement across the development organization. Further, the initiative should have executive sponsorship across the entire organization, as this should be viewed as a business transformation and not a departmental project. Some common characteristics have been identified for successful transformation initiatives.
First, it is important to model the current R&D process and how it impacts clinical supplies. Understanding the functional requirements of each of the “swim lanes” and the inter-relationship across these constituents will define challenging areas to focus on for initiating this activity. A template of common drug development activities and constituents supporting this activity provides a starting point for many organizations beginning a business transformation process. (Figure 7).
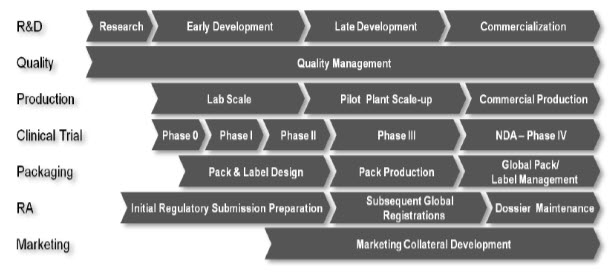
Figure 7 R&D Pipeline Cross Function Participants
Second, with a common development model outlined for the enterprise and key challenges identified, a business case can be developed to prioritize on which initiatives are most critical to address. A business case also helps justify the investment and provides metrics to measure realized business improvements such as ROI and operational performance for each specific initiative.
Third, for the initiatives that have been prioritized by the development challenges and justified with a business case, specific transformation requirements need to be defined. To define requirements, a review of the current IT landscape supporting these activities and the content managed in various systems must be compiled. This activity will establish base line processes to improve and help define requirements, plus define historical content that needs to be migrated/integrated to support the transformation.
The final step is to define a deployment plan to manage the transformation of these key objectives. It is critical that functional constituents who own these business processes are members of the deployment team in addition to executive sponsorship across the organization. With a well-represented cross-functional team supporting the transformation, concrete objectives will be defined and expectations clearly outlined for success. This approach also provides the foundation for “think big - start small – scale fast” for ongoing enterprise transformation.
SELECTION OF LCM TACTICS IN PHARMACEUTICALS
1. New formulation
2. Pediatric market exclusivity
3. Disease management programs
4. Strategic price change.
5. Authorize generics.
6. Combination products.
7. Next-generation product
8. New dosing regimen
9. Patent litigation
10. Rx to OTC switches
STRATEGIC BUSINESS PROCESSES REQUIRED TO SUPPORT TRANSFORMATION
Several companies are actively pursuing transformation initiates to address these challenges. Benchmarking of these initiatives helps identify common business processes required to enable this transformation. Based on input from 15 leading pharmaceutical companies who are members of the Oracle Pharmaceutical Strategic Council, seven common enabling elements have been identified to support this transformation. These 7 elements are as follows.
1. DRUG DEVELOPMENT PORTFOLIO MANAGEMENT.
The complexity of individual drug development programs is further complicated as there are typically multiple programs occurring simultaneously in the R&D pipeline at any given time. Traditional drug development program management has focused on general program metrics such as schedule, and cost performance. To improve drug development execution, program management that synchronizes cross-functional collaboration and archiving of critical program deliverables is required. Integration of the decisions and approvals of these deliverables provide the regulatory evidence needed to confidently advance the drug development process through each phase. Management of each program and required deliverable evident in one system can also enable standardization of best practices to improve pipeline performance.
2. STRUCTURED ELECTRONIC DRUG DEVELOPMENT RECORD (eDDR).
With the volume of program deliverables and regulatory evidence required in the development process ever increasing, a structured drug development archive is needed to effectively manage all of the associated content. This highly iterative development record must be automated to capture historical developmental information to support product claims and regulatory audits. Creating a structured archive for each individual design dossier in one unified system can also facilitate re-use of the Common Technical Documents (CTD).
3. INTIGRATED CLINICAL SUPPLY CHAIN MANAGEMENT.
Clinical trial management is a pivotal phase in the drug development process that has become more complicated with global and adaptive trials. As companies look for ways to reduce costs while significantly increasing profitability, externalization of clinical trials to CROs for example has become increasingly common and impacts everything from pre-clinical to post marketing research. The supply chain for clinical trials has also become increasing complex with multiple manufacturing sites and CMOs engaged in supporting the scale up for the required clinical inventory. For all supplies dispensed to each clinical site, a complete lot history of the campaigns producing the product must be archived. Synchronizing the approved manufacturing evidence of the clinical supplies with trial activity is required for clinical trial integrity to be maintained.
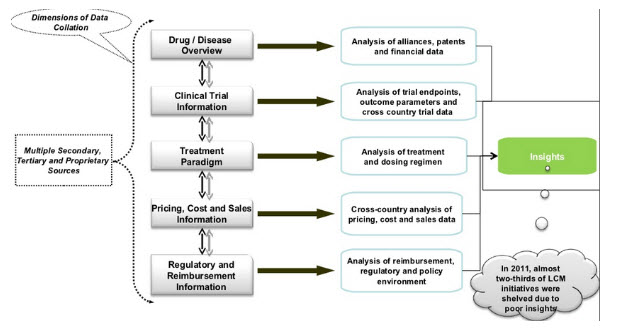
Figure-8 integrated supply chain
4. TECHNOLOGY TRANSFER AND COLABARATION.
The production of drug product is highly iterative and controls must be established for each lot from scale-up through commercialization of the final approved product. Effective scale-up of drug production requires collaboration across many interrelated activities and dependences. An enterprise solution that enables the analysis of the drug product value chain including suppliers, materials, equipment, and processes will not only provide individual lot control but also facilitate the scale-up to commercial drug production volumes.
5. INTEGRATED QUALITY AND RISK MANAGEMENT.
Quality and risk management continues to be a challenge creating significant business impact when deficiencies are indentified during regulatory audits. Furthermore, with the industries transformation to QbD practices for product development, the need for an Enterprise Quality Management (EQM) solution is further justified. An effective EQM solution requires the integration and management of quality beginning at product development through commercialization. Early awareness of quality events and immediately assessing the impact across the enterprise will provide the foundation to leverage quality management resulting in improved business performance.
6. COMPHRENSHIVE PACKAGING AND CO-LATERAL MANAGEMENT
The integration of packaging, labeling and associated marketing collateral into the drug development process provides a significant business opportunity in the pharmaceutical industry. Often the creation of these assets is deferred until late in the drug development cycle creating delays in market launch and increased cost. This disconnect from the drug development evidence also creates the potential for misleading off-label claims that can be devastating for a product. Creation of a global repository for all packaging components, digital assets such as logos and artwork, and marketing collateral that references development evidence will improve the regulatory integrity of all the associated commercial content. Re-use of this commercial product content, and common translation services are just a few of the business benefits companies like GSK and Bayer have realized by the enterprise management of this critical asset.
7. GLOBAL PRODUCT REGISTRATION.
The ultimate successful outcome of any drug development program is regulatory submission and approval for commercial distribution of the product. Global product registration is complex and constantly evolving, making registration management increasingly difficult. As a result, this creates delays in market launch and significantly impacts the anticipated product revenue. Leveraging the evidence captured in the previously described use cases provides the content to support regulatory submittal requirements. This content also can be used for on-going global product proliferation through re-use of this registration process significantly improving ROI for each new product developed.
CONCLUSION:
Increasingly, pharmaceutical companies seek to have a unified view of their entire product development lifecycle with the ability to view and trace every product detail throughout the entire process. Agile Product Lifecycle Management provides the capability to both manage and centralize product information, helping pharmaceutical companies realize their IT investments by addressing some of the most essential needs including speeding time to market, lowering overall operating and production costs and realizing quality standards such as QbD. Finally, by providing a unified view of the product(s) across the organization, companies can finally realize the benefits of cross-functional collaboration where product knowledge is transparent thus facilitating in product governance both internally and externally (i.e., compliance with regulatory agencies).In this white paper we have outlined 7 of the most essential steps in which pharmaceutical companies can approach PLM and lead the way towards a transformation of the entire business. Deployed properly, Oracle’s Agile PLM Software solutions for the Pharmaceutical industry can deliver rapid ROI by realizing productivity gains while reducing time to market and associated product development costs. These not only illustrate how transformational elements can improve the “lab to launch” process but also create a company culture for continuous improvement based on the mantra of “think big - start small - scale fast”
It is not the strongest of the species that survive
Nor the most intelligent,
But the one most responsive towards change.
Charles Darwin
REFERENCES:
1. Day, Martyn (15 April 2002). What is PLM. Cad Digest. Retrieved 25 February 2012.
2. Todd Hein, Michael Winkler, Hardeep Gulati, Arvindh Balakrishnan “Product Lifecycle Management for the Pharmaceutical Industry” Oracle Life Sciences.Page-1-2.
3. Dennis Z. Kvesic “Product Lifecycle Management: Marketing Strategies for the Pharmaceutical Industry” Journal of Medical Marketing: Device, Diagnostic and Pharmaceutical Marketing September 2008 vol. 8 no. 4 293-301.
4. Vandana Prajapati ,Harish Dureja “Product lifecycle management in pharmaceuticals” Journal of Medical Marketing: Device, Diagnostic and Pharmaceutical Marketing August 2012 vol. 12 no. 3 150-158.
5. Acito, Franklin and Arun K. Jain, "Evaluation of Conjoint Analysis Results: A Comparison of Methods", Journal of Marketing Research, 17, pages 106-112, 1980
6. Philip Needleman, “From a Twinkle in the Eye to a Blockbuster Drug”, Research-Technology Management, 2001.
7. Kevin O’Marah, “Product Lifecycle Management: What’s Real Now”, AMR Research, 2002.
8. Venkat Venkatsubramanian, “Prognostic and Diagnostic Monitoring of Complex Systems for Product Lifecycle Management: Challenges and Opportunities”.
9. Ioannis Komninos, “Product Lifecycle Management”, Urban and Regional Innovation Research Unit, Aristotle University of Thessaloniki.
10. Rebecca S. Yoshitani, J.D. and Ellen S. Cooper, J.D “pharmaceutical reformulation: the growth of life cycle management” Houston Journal of Health Law & Policy 7 HOUS. J. HEALTHL. & POL’Y379–410
11. Mark D. Shtilerman, Pharmaceutical Inventions: A Proposal for Risk-Sensitive Rewards, 46 IDEA 337, 348–50 (2006);
12. Robert H. Lande, Consumer Choice as the Ultimate Goal of Antitrust, 62 U. PITT. L. REV 503, 503–04 (2001)
13. Hill, Sidney (September 2006). "A winning strategy". Manufacturing Business Technology. Retrieved 25 February 2012.
14. Gould, Lawrence (12 January 2005). "Additional ABCs About PLM". Automotive Design and Production. Retrieved 25 February 2012.
15. Gould, Lawrence (12 January 2005). "Additional ABCs about PLM". Automotive Design and Production. Retrieved 25 February 2012.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









