{ DOWNLOAD AS PDF }
ABOUT AUTHORS
*Nayak Chandan1, PadhiPriyanka1
Gayatri Institute of Science and Technology,
Gunupur Odisha, India
*nayakchandan279@gmail.com
ABSTRACT:
The study is concerned with the development of Ethyl Cellulose microspheres by the o/w emulsification and solvent evaporation method in the presence of tween 80 as an emulsifying agent. The influence of process parameters such as solvent mixture, composition, concentration of the emulsifying agent and speed of stirring has been examined. The microspheres have been analysed for their size, drug loading capacity and drug release study. Spherical and smooth surfaced microspheres with desired encapsulation efficiencies were obtained. Slow drug release from microspheres observed up to6- 8 h. An optimization procedure was employed to investigate and identify the key parameters affecting the properties of the microspheres.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2512
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 4 Received On: 16/04/2017; Accepted On: 19/03/2018; Published On: 01/04/2018 How to cite this article: Nayak C, Padhi P; Preparation and Characterization of Ethyl Cellulose based Salbutamol Sulphate and Theophylline Combination Microsphere; PharmaTutor; 2018; 6(4); 51-69; http://dx.doi.org/10.29161/PT.v6.i4.2018.51 |
INTRODUCTION:
For many decades, acute diseases or a chronic illness has been treated and managed by conventional pharmaceutical dosage forms like tablet, capsules, liquids and semi-solid preparation. Microspheres are one of the multiparticulate drug delivery systems and are prepared to obtain prolonged (or) controlled drug delivery, to improve bioavailability or stability and to target drug to specific sites. Microspheres can be defined as solid, approximately spherical particles ranging from 1 to 1000µm, containing dispersed drug in either solution (or) microcrystalline form. Ethyl cellulose is non-biodegradable, bio-compatible, non-toxic natural polymer and widely used in oral and topical formulation. The microspheres can be produced by several methods utilizing emulsion system (o/w, w/o, o/w/o and w/o/w). The common emulsion system used oil-in-water (o/w), with microspheres being produced by the emulsion solvent evaporation method. This relatively simple method enables the entrapment of a wide range of hydrophobic drugs.4 The main object of present study was to investigate the possibility of obtaining sustained release aspirin microsphere by using different concentration of ethyl cellulose, different composition of solvent mixture, different concentration of emulsifying agent and different stirring rate. Some of the basic factors to be taken in an account while designing a controlled release drug delivery system includes:
* Effective therapeutic concentration of the drug
* The release kinetic and mechanism of drug release from the device
* The physico-chemical properties of the drug as well as the delivery device
MATERIALS:
Salbutamol sulphate as a model drug, Ethyl Cellulose as a polymer, Tween80 and span 80 as an Emulsifying agent, Acetone and Ethyl Acetate as a solvent were used for present investigation. All the materials were provided by G.I.S.T COLLEGE, GUNUPUR
METHODS:
Preparation of Microspheres: Microspheres were prepared based on oil-in-oil emulsion solvent evaporation technique by using the formulation. In this method 900mg of ethyl cellulose was dissolved in 15 ml of acetone and a given amount of the drugs were dispersed in it to make different drug to polymer ratio 1:1,1:5,1:2 for salbutamol sulphate microspheres and 1:1,1:2,1:3 for combination microspheres and stirred for about 10minut .then the polymer drug dispersion was poured into 50ml of liquid paraffin(light)containing varying of dispersing agents which are dispersed and stirred for about 30 minute before the polymer –drug dispersion was added. The whole systems was then stirred for about 4hour at 900rpm.after stirring process is over the liquid paraffin (light) was decanted off and microcapsule formed were collected and washed with cyclohexane to completely remove the reaming oil and dried at 60˚C in vacuum drier for 6 hour and collected for further studies and characterized.
Physicochemical Characterization of Microsphere
Percentage yield : The dried microspheres were weighed and percentage yield of the prepared microspheres was calculated by using the following formula, Percentage yield = {the weight of microspheres / (The weight of polymer + drug)}*100
Carr’s index
It was measure by using following formula,
Carr’s Index = {(Vb –Vt) / Vb}* 100
Where, Vb and Vt are the bulk volume and tapped volume respectively.
Angle of Repose
Angle of repose of the microspheres, is the maximum angle possible between the surface of the pile of microspheres and the horizontal plane, was obtained by fixed funnel method using the formula; Angle of Repose (θ) = tan−1 (h/r ) Where, h is height and d is the diameter of the microsphere pile.
Particle Size Analysis
Particle size of the microspheres was determined by optical microscopy. The eye piece micrometre was calibrated with the help of a stage micrometre. The particle diameters of more than 50 microspheres were measured randomly. The average particle size was determined by using Edmondson’s equation. D = ∑ nd / ∑ n Where, n = Number of microspheres checked; D = Mean of the size range.
FORMULATION OF SALBUTAMOL SULPHATE MICROSPHERE:
Different drug to polymer ratios as well as different concentration (%v/v in light mineral oil) of emulsifying agent was taken to study their effects on different properties of the resultant microsphere. Formulation variables are listed in details in the following tables:
Table-1
FORMULATION USING TWEEN 80:
|
FORMULATION CODE |
DRUG:POLYMER |
AMOUNT OF POLYMER(mg) |
AMOUNT OF SALBUTAMOL SULPHATE(mg) |
PERCENTAGE OF DISPERSING AGENT (%) |
|
A1 |
1:1 |
900 |
900 |
0.2 |
|
A2 |
1:1 |
900 |
900 |
0.6 |
|
A3 |
1:1 |
900 |
900 |
1 |
|
B1 |
1:1.5 |
900 |
600 |
0.2 |
|
B2 |
1:1.5 |
900 |
600 |
0.6 |
|
B3 |
1:1.5 |
900 |
600 |
1 |
|
C1 |
1:2 |
900 |
450 |
0.2 |
|
C2 |
1:2 |
900 |
450 |
0.6 |
|
C3 |
1:2 |
900 |
450 |
1 |
Table-2
FORMULATION USING SPAN 80:
|
FORMULATION CODE |
DRUG:POLYMER |
AMOUNT OF POLYMER(mg) |
AMOUNT OF SALBUTAMOL SULPHATE(mg) |
PERCENTAGE OF DISPERSING AGENT (%) |
|
D1 |
1:1 |
900 |
900 |
0.2 |
|
D2 |
1:1 |
900 |
900 |
0.6 |
|
D3 |
1:1 |
900 |
900 |
1 |
|
E1 |
1:1.5 |
900 |
600 |
0.2 |
|
E2 |
1:1.5 |
900 |
600 |
0.6 |
|
E3 |
1:1.5 |
900 |
600 |
1 |
|
F1 |
1:2 |
900 |
450 |
0.2 |
|
F2 |
1:2 |
900 |
450 |
0.6 |
|
F3 |
1:2 |
900 |
450 |
1 |
Table-3 FORMULATION OF COMBINATION:
|
SL NO |
FORMULATION CODE |
AMT OF POLYMER (mg) |
AMT OF ANHYDROUS THEOPHYLLINE (mg) |
AMT OF SALBUTAMOL SUPHATE(mg) |
PERCENTAGE OF TWEEN 80(%V/V) |
RPM |
|
1 |
T1 |
1219.2 |
1200 |
19.2 |
1 |
900 |
|
2 |
T2 |
1219.2 |
600 |
9.6 |
1 |
900 |
|
3 |
T3 |
1219.2 |
400 |
6.4 |
1 |
900 |
PARTICLE SIZE DETERMINATION OF MICROSPHERE
The particle size of the microspheres was determined by microscopic method (72, 73).the ocular micrometre was calibrated using stage micrometre and each division of the ocularmicrometre was measured in micrometre.For each batch of the microsphere, 100 particles were counted and done in triplicate. Calibration of ocular micro meter: 1 division of ocular micro meter =15µm.
DRUG ENTRAPEMENT EFFICIENCY:
The amount of salbutamol sulphate present in the microsphere was determined by extraction in distilled water (48). 50 mg of the crushed and powdered microsphere was taken and extracted in 50 ml of distilled water and stirred for 15 minute at 1500rpm. The solution was filtered and after suitable dilution the content of salbutamol sulphate was determined spectrophotometric ally at 276nm. The same also applied for combination microsphere. Drug entrapment efficiency =experimental drug content /theoretical drug content ×100
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
IN-VITRO DRUG RELEASE STUDY:
THE IN-VITRO RELEASE STUDY of the microsphere was carried out using rotating basket method at 50 rpm at 37℃. Dissoulation study was performed in 3 different media (distilled water, phosphate buffer pH 7.4 and 0.1 M HCL) for salbutamol sulphate microsphere taking 900 ml for each study. For combination microsphere were,dissolution medium was only phosphate buffer of pH 7.4,50 mg of the microspheres were taken for dissolution in salbutamol sulphate microsphere while 100 mg of microsphere were taken in case of combination microspheres due to low concentration of salbutamol sulphate in the microspheres. 5 ml of the sample were withdrawn at a pre-set time interval for salbutamol sulphate microspheres and analysed spectrophotometric ally at 276 nm.1 ml of sample was withdrawn in case of combination microspheres and diluted 100 times with 0.05 M NaOH and analysed spectrophotometric ally (64). The release kinetics was then studied.
INFRARED SPECTROSCOPY:IR SPECTRA OF SALBUTAMOL SULPHATE (pure drug and microsphere), theophylline (pure drug) and combination microsphere as well as blank microsphere were recorded using PerkinElmer model 883 IR-spectra photo meter between the range of 500to 4000cminverse. The resultant spectra were then compared with standard reference (IP 1996) and above observe for any type of deviation from the standard.
SCANNING ELECTRON MICROSCOPY (SEM):
Scanning electron microscopy was done to characterize surface topography of the microspheres. Photomicrograph of the microspheres before and after the release of the drugs was taken. The quality of the microspheres (with respect to surface properties) and the changes that occur during in-vitro dissolution studies may have implication to the performance of the microspheres.
RESULTS AND DISCUSSION: Microsphere was prepared by using ethyl cellulose by employing emulsification solvent evaporation technique. Salbutamol sulphate was used as a model drug to check the drug entrapment efficiency and drug release study. The prepared microsphere was evaluated to check the effect of key parameter affecting on the properties of microsphere.
CHARACTERIZATION OF THE DRUG: The gifted sample of theophylline (anhydrous) and salbutamol sulphate received were characterized by physical observation, by determining their melting points and saturation solubility at different solvents and by recording their ultraviolet (UV)and infrared (IR)absorption spectra which were compared and matched with reference spectra.
Melting point: M.P of the theophylline sample was found to be 272℃ and it matches with the pharmacopeiaspecificationconfining the identity of the received sample.M.P of salbutamol sulphate is specified in the pharmacopeia, but observation revels that it starts melting at 196℃.
Characterization of the drugs: Table-4
|
Characteristic Drugs |
Appearances |
Colour |
Melting point |
Saturation solubility |
||||||
|
Observed |
I.P. Specifications |
Observed |
I.P. Specifications |
Observed |
I.P. specifications |
Distilled water |
Phosphate buffer |
|
||
|
Anhydrous theophylline |
Crystalline powder |
Crystalline powder |
White
|
white |
271 |
270-274 |
14.8±0.82 mg/ml |
10.8±0.326 mg/ml |
|
|
|
Salbutamol sulphate |
Crystalline powder |
Crystalline powder |
White |
White or almost white |
196 |
- |
650±0.46 mg/ml |
545.5±0.88 mg/ml |
|
|
INFRARED (IR) SPECTRA:IR spectra of theophylline (anhydrous) and salbutamol sulphate were compared with that of reference spectra. The spectra of the samplewhen compared with reference show similar peaks as shown in figure,
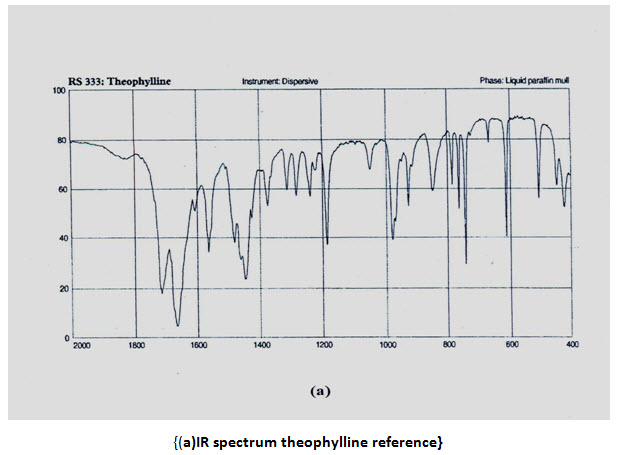

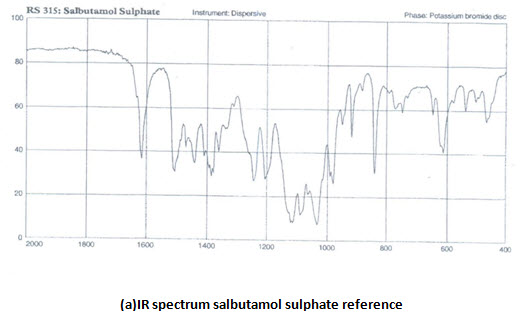

Ultraviolet spectra and absorption maxima:
UV absorbance of 0.001%w/v solution of theophylline in 0.1 M HCL at 270 nm is 0.730 [IP limit is –not less than 0.53]. UV scans in the wavelength range of 400-200 nm shows λmaxat 270.5 nm. UV absorbance of 0.008% w/v salbutamol sulphate solution in 0.1 M HCL at276 nm is 0.434 [IP range is 0.53-0.60]. UV scans showsλmaxat 276nm. The UV analysis revels ahigh purity of the samples
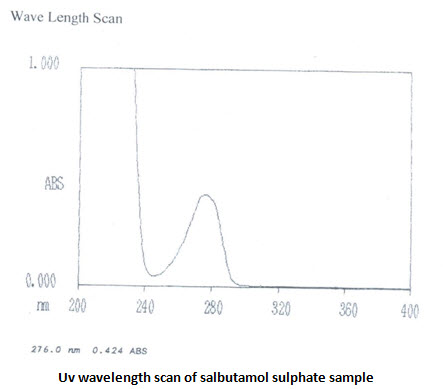
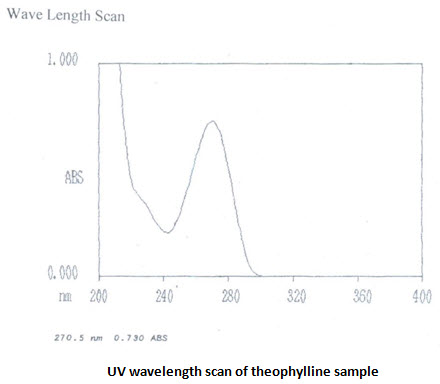 Calibration curve:
Calibration curve:
Calibration curve of both drugs was prepared in phosphate buffer pH 7.4. The concentration was increased in a predetermined way. Regression equation was calculated and utilized for quantitive estimation of drugs. The correlation found were given
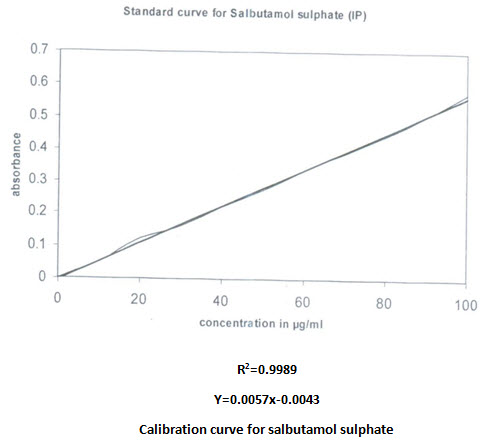
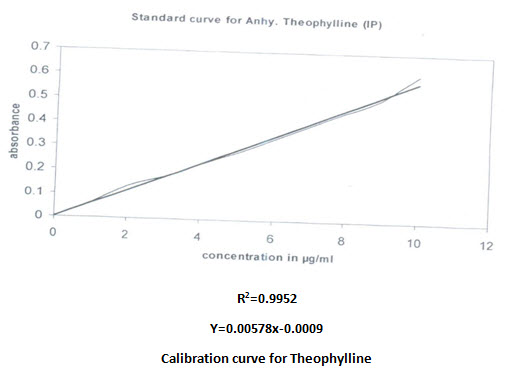
Validation of UV spectroscopic method: Simultaneous equation are solved with absorbance obtained at 244.6nm and 274.6nm.the concentration obtained (moles/litre) were converted into amount (gram/litre), the accuracy of the method was found to be high enough for routine analysis as shown in the following table.
Table-5
|
DRUGS |
AMOUNT TAKEN (MG) |
PERCENT ESTIMATED( ) |
|
Theophylline |
420 |
97.87 |
|
Salbutamol sulphate |
10 |
95.44 |
Viscosity of polymer: The viscosity of 5%w/v solution of ethyl cellulose in 80:20 toluene: ethyl alcohol, determined at capillary viscometer was found to be 29 cps at room temperature (28℃).the authors found that the higher the viscosity grade of the polymer, the slower the release rate of the drug from the film until the critical viscosity is reached where there is no further decrease of release rates. This is due to increase in polymer molecular weight.
PARTICLE SIZE: The mean particle size of the formulations were found in between 500nm and 1400nm as shown in table, the mean particle size distribution was found to be affected by variable taken. Similar types of effects are obtained in salbutamol sulphate loaded ethyl cellulose microspheres as well as salbutamol sulphate-theophylline combination loaded microspheres.
Table:6
(Particle size of different formulation of salbutamol sulphate microsphere)
|
Formulations of microspheres (drug: polymer) |
Conc.of dispersing agents(%v/v) |
Mean particle size of tween 80 microsphere(µm) |
Mean particle size of span 80 microspheres(µm) |
|---|---|---|---|
|
1:1 |
0.2 |
1426.59±6.54 |
886.69±1.159 |
|
1:1 |
0.6 |
941.487±3.85 |
762.33±2.635 |
|
1:1 |
1 |
872.92±2.673 |
586.205±2.197 |
|
1:1.5 |
0.2 |
1363.95±4.237 |
732.53±2.74 |
|
1:1.5 |
0.6 |
934.73±4.106 |
725.78±1.125 |
|
1:1.5 |
1 |
856.85±5.95 |
684.24±1.681 |
|
1:2 |
0.2 |
1267.77±8.94 |
633.21±2.57 |
|
1:2 |
0.6 |
875.22±2.725 |
490.7±1.752 |
|
1:2 |
1 |
681.23±3.86 |
450.79±1.54 |
(Mean particle size of different formulation of salbutamol sulphate and anhydrous Theophylline combination microspheres)
|
Formulation codes |
Mean particle size(µm) |
|---|---|
|
T1 |
730.55±1.582 |
|
T2 |
695.64±2.565 |
|
T3 |
689.71±1.281 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Effects of types of surfactants on particle size:
Two types of surfactants used have an influence on the particle size distribution of the microspheres. The hydrophobic surfactant Span 80 is found to produce smaller particle size microspheres compared to hydrophobic surfactant tween 80. Span 80 is oil soluble and produces a stable w/o type emulsion. This may explain why smaller particle size are obtained with span 80.there is better incorporation of span 80 due to its oil solubility and resulted in better emulsification

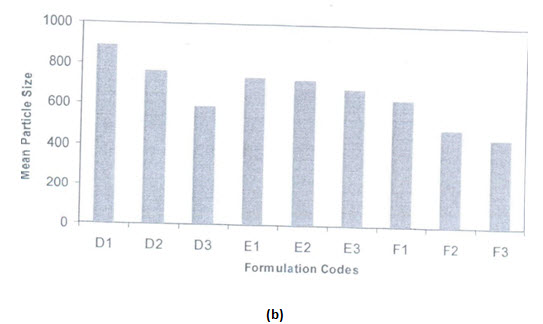
(a) Effect of tween 80 concentration on microsphere size distribution
(b) Effect of span 80 concentration on microsphere size distribution
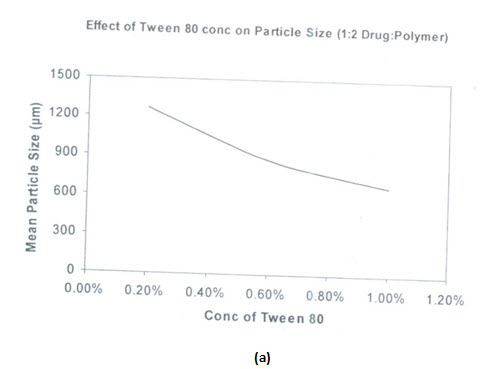

(a)Effects of span 80 concentration on particle size of salbutamol sulphate microspheres
(b)Effect of span 80 concentration on microsphere size distribution
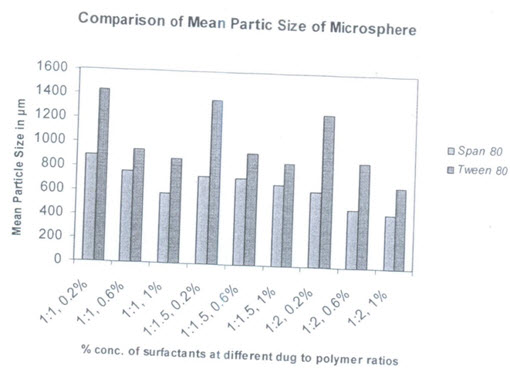
(Comparison graph of mean particle size of salbutamol sulphate microspheres from tween 80 and span 80 surfactants with different concentration)
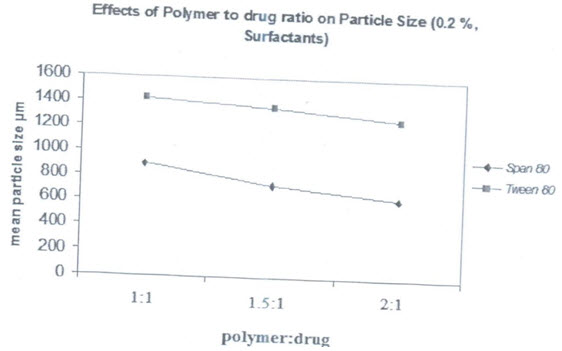
(Effects of polymer :drug ratio on mean particle size of salbutamol sulphate microspheres prepared from both types of surfactants with concentration0.2%)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SCANNING ELECTRON MICROSCOPY:
Scanning electron microscopy of drug loaded ethylcellulose microspheres reveals that the microspheres posses a rough and rugged surface. Magnification of the microspheres to 5000 times shows that the surface cantains some crystals deposited in it, which probably is a drug that is required for initial brust release. The micrograph taken after 12 hours release studies also reveals porosity developed at the surface. The surface porosity is crucial for drug release in microspheres prepared with ethylcellulose. Since the polymer is not biodegradable, the release of the drug from microspheres takes place by dissolution and diffusion through the pores. Ethylcellulose allows water to permeate through its surface without itself dissolving in it. The porosity may also indicate the wettability of the microsphere surface due to the incorporation of surfactents. The size and number of the pores and surface wettability determines the rate and extent of release from the microspheres.Some of the micrographs taken are presented in the following figure. The figure show that porosity developed but structure is retained as 12 hours drug release study.

(Salbutamol sulphate microsphere prepared before drug release with different magnifications)
DRUG ENTRAPMENT EFFICIENCY:
The entrapment efficiency was determined at phosphate buffer of pH 7.4 . higher percentage entrapment was found when the percentage of surfactant was increased from 0.2% to 1%. This is true in both types of surfactants used. The effect of increased in polymer concentration on drug entrapment is not indicateed significantly in the results.
Table-7 :percentage entrapment of salbutamol sulphate
|
Formulation codes |
Percent entrapped |
Formulation codes |
Percent entrapped |
|
A1 |
86 |
D1 |
84 |
|
A2 |
84 |
D2 |
90.8 |
|
A3 |
92 |
D3 |
94 |
|
B1 |
84.5 |
E1 |
94.5 |
|
B2 |
87.5 |
E2 |
89.5 |
|
B3 |
87.22 |
E3 |
82 |
|
C1 |
80.98 |
F1 |
84 |
|
C2 |
80.98 |
F2 |
94.5 |
|
C3 |
83.98 |
F3 |
90 |
Percentage entrapment of salbutamol sulphate theophylline in combination microspheres:
|
Formulation codes |
Percent salbutamol sulphate entrapped |
Percent theophylline entrapped |
|
T1 |
78.667±2.45 |
82.865±1.75 |
|
T2 |
76.534±3.67 |
86.789±0.986 |
|
T3 |
83.45±1.25 |
83.468±1.32 |
IR ANALYSIS: The IR-spectrum of salbutamol sulphate showed sharp peaks at 1100 cm-1(c-o stretch) and about 1500 cm-1 for OH bending. The identical peaks were also present in salbutamol sulphate loaded ethyl cellulose microspheres. The IR spectrum of theophylline showed peaks at 1650 cm-1 for –NH bending. Peaks are also obtained at 1450 cm (-CH bending in plane) 1170 cm-1 for C-C stretch .all the peaks are also obtained in the microspheres, conforming their compatibility.
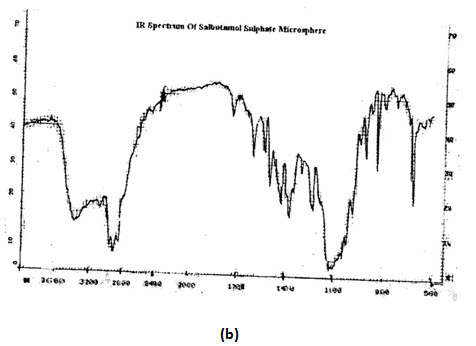
SALBUTAMOL SULPHATE MICROSPHERS:
The in vitro release of the drug from ethyl cellulose microspheres is found to be biphasic with the initial burst effect which was followed by much slower gradual release of the drugs. The initial burst release may be due to presence of drug particles on the surface during encapsulation. The amount of drugs released by the initial burst effect varies with batches and upto 70-80 % of the drugs can be released during the initial release phase and this phase may extent upto 2 hours. The initial burst release is seen in almost all the batches. It is found to be advantageous since it will provide for the achievement of initial therapeutic concentration. The decrease in release rate is due to the exhaustion of the drugs on the surface and the increase in diffusional path-length as the drug continues to dissolve and the diffuse from the matrix. There are certain factors that affect the rate and extent of drug release from the matrix microspheres. Since the release of the drugs from control release device is correlated with the performance and effectiveness of the device as a controlled release system, formulation factors are varied to optimise the in-vitro drug release.
Effect of surfactant concentration on in-vitro salbutamol sulphate release:
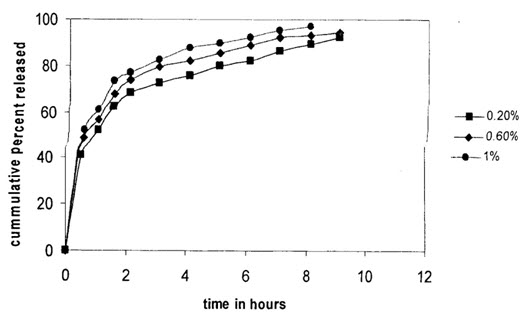
Effect of Tween 80 concentration on drug released (polymer: drug ratio is 1:1)
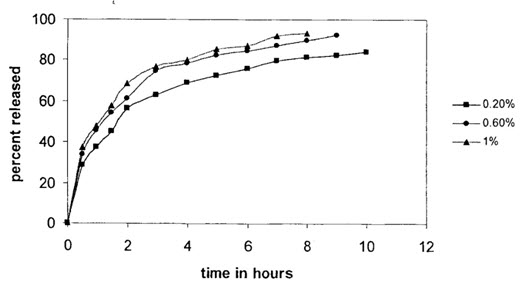
Effect of span 80 on drug release (1:1 polymer: Drug)
Effect of surfactant type on in-vitro salbutamol sulphate release:
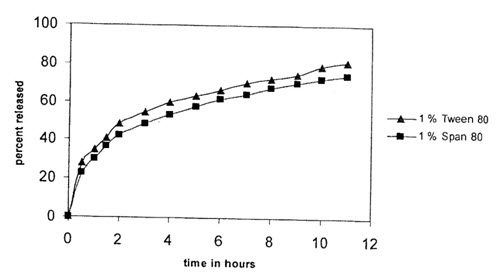
(Comparison graph for percentage released from two surfactant (1:2, drug: polymer)
Effect of drug to polymer ratio on in-vitro drug release:
The effects of drug to polymer concentration on in vitro release was studied. Drug to ethyl cellulose ratio taken were 1:1, 1:1.5 and 1:2 for salbutamol sulphate microspheres. The increase in polymer concentration resulted in decrease of drug release. The decrease in drug release rate with increase in polymer concentration is due to the increase as the polymer concentration is increased
Effect of dissolution medium on drug release:
Dissolution study of salbutamol sulphate microspheres was carried out on different dissolution media, distilled water, 0.1M HCL and phosphate buffer of pH 7.4. The release rate was found to be affected remarkably. Dissolution data obtained shows more or less similar release kinetics. This type of finding was also reported by Adelsakr et al. this shows that drug release from ethyl cellulose is not affected by dissolution media pH, but the solubility of drug on the media.

(Effect of dissoulation medium on the release of salbutamol sulphate (1:2, drug:polymer,1%tween 80)
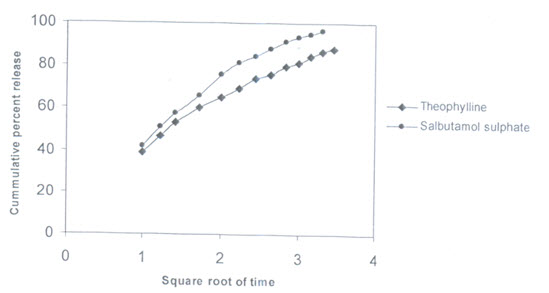
THEOPHYLLINE MICROSPHERES:
Theophylline microsphere was prepared with 1:2 drug:polymer ratio using 1% tween 80 surfactant and the in-vitro release was studied on phosphate buffer of pH 7.4.
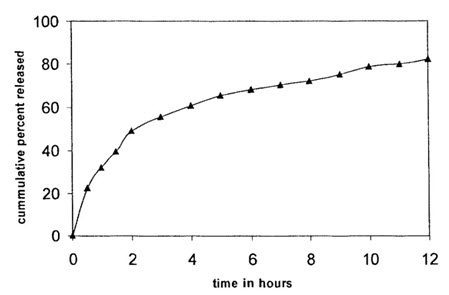
(Theophylline release from theophylline microsphere)
SALBUTAMOL SULPHATE AND THEOPHYLLINE COMBINATION MICROSPHERES:
Effects of drug to polymer ratio on in-vitro drug release:
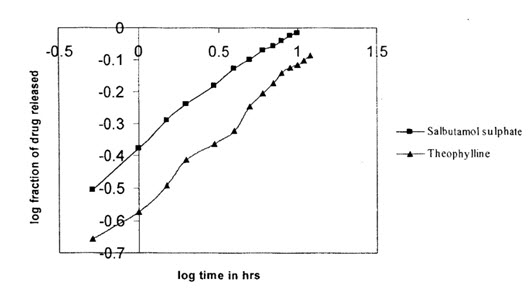
The effects of variation in drug to polymer ratio was on drug release was also studied on Salbutamol sulphate and Theophylline combination microspheres in a dissolution medium of phosphate buffer pH 7.4.Different drug to polymer ratio taken were 1:1,1:2 and 1:3. As in the case salbutamol sulphate microspheres,increases in polymer concentration resulted in a decrease of drugs release.the release of salbutamol sulphate was found to be much higher as compared to theophylline release, this may be due to thehigher solubility of salbutamol sulphate than theophylline in the dissolution medium.
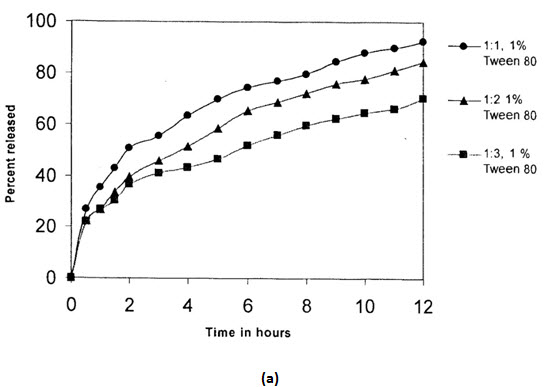
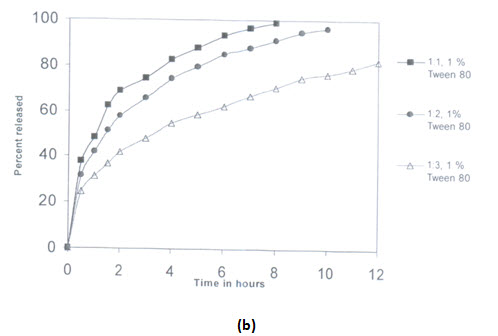
(Release from combination microspheres of different drug:polymer ratios
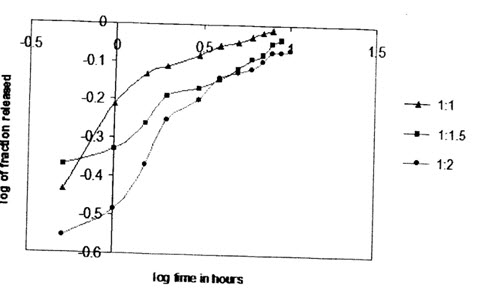
(Higuchi plot of combination microsphere drug release (1:2,drug: polymer,1% tween 80)
Study of effectiveness of matrix combination microspheres:
The effectiveness of the combination was studied in its in-vitro release.salbutamol sulphate and Theophylline microspheres were prepared separetely and mixed in a ratio to simulate the drugs ratio obtained in the combination matrix.In-vitro release was studied in phosphate buffer pH 7.4.It significantly,there is a decrease in Theophylline release from combination matrix compared to microspheres mixed.This may be due to the increase in surface area when separate Theophylline microsphere is taken.

(Comparison of drug release from combination matrix and separate microspheres of salbutamol sulphate and theophylline taken in the ratio to stimulate the ratio in combination matrix.the drug:polymer ratio is 1:2 with 1% tween 80)
RELEASE KINETICS:
In order to describe the kinetics of the release process of drugs from controlled release preparation,the data were fitted with different kinetics models.The first order equation describes the release from systems when dissolution rate is dependent on the concentration of the dissolving species.The Higuchi square root equation describes the release from systems where the solid drug is dispersed in an insoluble matrix and the rate of drug release is related to the rate of of drugs from ethylcellulose microspheres is found to be diffusion controlled release.In order to determine the model which will represent a best release kinetics model for the prepared formulations,the dissolution data was analyzed using the peppas and korsenmyer equation which is expressed as Mt/M∞=k.tn Where mt is the amount of drug release at time t and M∞ is the amount release at time t=∞,thusMt/M∞ is the fraction of drug release at time t,k is the kinetics constant,and n is the diffusion exponent which can be used to characterize both mechanism for both solvent penetration and drug release.These values fall in the region of 0:5<n<1:0,which corresponds to a Fickian and case diffusion dependent release which is refferred to as anomalous diffusion.The goodness of the fit is shown through detemination of correlation coeffcient.Very high correlation coefficient (close to 1)for a particular kineitics model indicates that the release can be charcterized by that model.The diffusionexponent,n,was found to be in between 0.2 to 0.5 which indicates that the release of drug from the microsphere controlled release.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Kinetic Table-1: salbutamol sulphate microspheres [1%,span 80]
|
Formulation (drug:polymer) |
Zero- order |
First order |
Higuchi model |
Korsenmeyerpeppas model
|
|||||||
|
r2 |
K0 |
r2 |
K1 |
r2 |
Kh |
r2 |
n
|
||||
|
1:1 |
0.6947 |
6.6512 |
0.9576 |
0.114 |
0.8995 |
26.552 |
0.994 |
0.4961 |
|||
|
1:1.5 |
0.8357 |
6.2262 |
0.9745 |
0.0698 |
0.9762 |
24.743 |
0.9878 |
0.3172 |
|||
|
1:2 |
0.8078 |
5.7496 |
0.9570 |
0.0589 |
0.9616 |
23.115 |
0.9979 |
0.2936 |
|||
Kinetic Table-2: salbutamol sulphate microspheres [1%,tween 80]
|
Formulation (drug:polymer) |
Zero- order |
First order |
Higuchi model |
Korsenmeyerpeppas model
|
|||||||
|
r2 |
K0 |
r2 |
K1 |
r2 |
Kh |
r2 |
n
|
||||
|
1:1 |
0.607 |
8.0134 |
0.9491 |
0.1841 |
0.9827 |
26.485 |
0.9588 |
0.5622 |
|||
|
1:1.5 |
0.7493 |
5.7121 |
0.9652 |
0.0808 |
0.9787 |
23.489 |
0.9827 |
0.5034 |
|||
|
1:2 |
0.8403 |
5.0517 |
0.9705 |
0.068 |
0.9709 |
25.092 |
0.8678 |
0.3173 |
|||
From the release kinetics table if can be observed that the release of salbutamol sulphate from the ethylcellulose microsphere exhibit diffusional characteristics and closely follows higuchi model and also highly correlated with first-order release model.
Kinetic table for release of drugs from combination microspheres:
(a)Theophylline Table-3
|
Formulation (drug:polymer) |
Zero- order |
First order |
Higuchi model |
Korsenmeyerpeppas model
|
|||||||
|
r2 |
K0 |
r2 |
K1 |
r2 |
Kh |
r2 |
n
|
||||
|
1:1 |
0.7406 |
6.9769 |
0.9707 |
0.104 |
0.9842 |
26.287 |
0.9907 |
0.2506 |
|||
|
1:2 |
0.7908 |
5.5131 |
0.9687 |
0.068 |
0.9523 |
23.334 |
0.9926 |
0.2798 |
|||
|
1:3 |
0.8461 |
4.4599 |
0.955 |
0.037 |
0.9782 |
18.44 |
0.9902 |
0.2334 |
|||
(b)Salbutamol sulphate: Table-4
|
Formulation (drug:polymer) |
Zero- order |
First order |
Higuchi model |
Korsenmeyerpeppas model
|
|||||||
|
r2 |
K0 |
r2 |
K1 |
r2 |
Kh |
r2 |
N
|
||||
|
1:1 |
0.767 |
9.5228 |
0.9579 |
0.213 |
0.9525 |
33.335 |
0.9811 |
0.4225 |
|||
|
1:2 |
0.7951 |
6.8991 |
0.9873 |
0.131 |
0.9879 |
27.889 |
0.9919 |
0.4447 |
|||
|
1:3 |
0.8671 |
4.8203 |
0.9636 |
0.043 |
0.9763 |
19.903 |
0.9973 |
0.3037 |
|||
The release of both salbutamol sulphate and theophylline from the microspheres was fitted into different release models, the release kinetic also exhibit diffusional characteristic and correlated well with higuchi model and first order release model.
(Koresenmeyer-peppas plot for tween 80 prepared salbutamol sulphate microspheres(1%) with different drug to polymer ratios)
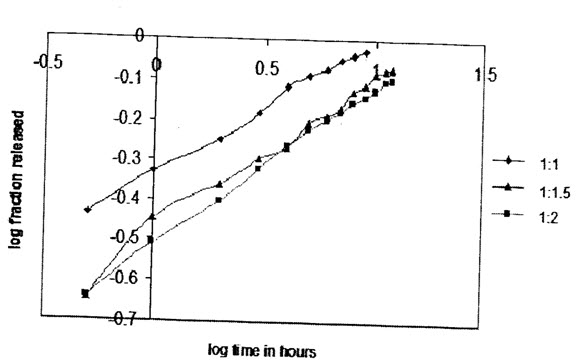
(Koresenmeyer-peppas plot for span 80 prepared salbutamol sulphate microspheres(1%) with different drug to polymer ratios)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION:
Matrix microspheres of salbutamol sulphate and theophylline combination were successifully prepared by emulsion solvent evapouration method. The microspheres were spherical in shape with rugged and pourous surface. The types and concentration of surfactants used were shown to have significant effects on different properties of microspheres and its release characteristic. Probaly the most important aspects when developing new pharmaceutical products or elucidating drug release mechanism is the developing new phrmaceutical products or elucidating drug release mechanism is the desired or required predictability and accuracy of the device.Non-biodegradeble matrix system showed a simple and established release pattern eventhrough zero-ordernrelease is not normally expected. Studies on the release kinetic showed that the release pattern was diffusional and follows higuchi model and also shows acloser relation with first-order release mechanism.
REFERENCES:
1. Ainley Wade & Paul J. Weller, Handbook of Pharmaceutical Excipients, 2nd edition, The Pharmaceutical Press, London, 1994; pp 186-190.
2. Das MK and Rao KR. Evaluation of zidovudine encapsulated ethyl cellulose microspheres prepared by water in oil in oil (w/o/o) double emulsion solvent diffusion technique. Acta Pol Pharm., 63(2); 141-148.
3. Gibaud S, Bonneville A, Astier A. Preparation of 3,4- diaminopyridine microparticles by solvent-evaporation methods. Int J Pharm., 242:197-201.
4. Hitesh K, Koshy MK, Shubhini AS. Gastroretentive Ethyl Cellulose Floating Microspheres containing Ranitidine Hydrochloride. Int. J. Drug Dev. & Res., 4(2); 315321.5. Kilicarslan M, Baykara T. The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int J Pharm., 252; 99-109.
6. Naveen C, Kirankumar Y, Rashmi V. Preparation and Evaluation of Ethyl Cellulose Microspheres Containing Diclofenac Sodium by Novel W/O/O Emulsion Method. J. Pharm. Sci. & Res., 2(12); 884-888.
7. Remington’s, The Science and Practice of Pharmacy, 21st edition, Volume–I, Lippincott, Williams and Wilkins, Philadelphia, 2006; pp 316.
8. Saravanan M, Dhanaraju D, Sridhar SK, Ramachandran S, Kishore GS, Anand P, Bhaskar K, Srinivasarao G. Preparation, Characterization and In vitro release kinetics of ibuprofen polystyrene microspheres. Ind.J.Pharm.Sci, 66 (3); 287-292
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









