{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Prajapati Krishna V*, Raj Hasumati A, Jain Vinit C, Prajapati Neelam S.
Department of Quality Assurance,
Shree Dhanvantary Pharmacy College, Kim, Surat, Gujarat, India
*krish1112k@gmail.com
ABSTRACT
This review article presents the pharmacology of combined Mesalazine and Rifaximin therapy especially in inflammatory bowel disease. Mesalazine is used as in anti-inflammatory agent, Non-Steroidal. Rifaximin is used in Gastrointestinal Agents, Anti-infective agent. The use of Rifaximin in combination with Mesalazine has been proved to provide beneficial effect in inflammatory bowel disease. The mechanism of Mesalazine and Rifaximin is quite different. Mesalamine and Rifaximin are two different types of drugs offering some symptomatic relief to the IBD patients. Mesalamine treats inflammation, whereas, Rifaximin reduces bio burden.
Patent for combination of both drugs were approved by WIPO. The main objective of this review article is to provide pharmacological information of combined therapy of Mesalazine and Rifaximin to researcher in development of combined dosage form of this.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2412
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 5 Received On: 04/01/2016; Accepted On: 21/01/2016; Published On: 01/05/2016 How to cite this article: Prajapati KV, Raj HA, Jain VC, Prajapati NS; Pharmacology of Combined Mesalzine and Rifaximin Therapy for Inflammatory Bowel Disease; PharmaTutor; 2016; 4(5); 41-45 |
INTRODUCTION [1-5]
Inflammatory bowel disease (IBD) is a spectrum of chronic idiopathic inflammatory intestinal conditions. IBD is a group of inflammatory conditions of the colon and small intestine. IBD causes significant gastrointestinal symptoms that include diarrhea, abdominal pain, bleeding, anemia, and weight loss. IBD also is associated with a spectrum of extra intestinal manifestations, including arthritis, ankylosing spondylitis, sclerosing cholangitis, uveitis, iritis, pyoderma gangrenosum, and erythema nodosum.

Figure 1: Inflammatory bowel disease [2]
Major types of IBD:
[1] Crohn's disease (CD):
CD is a condition of chronic inflammation potentially involving any location of the GIT from mouth to anus. CD is nonspecific inflammatory bowel disease that may affect any segment of the gastrointestinal tract. Crohn's disease, by contrast, is characterized by Trans mural inflammation of any part of the gastrointestinal tract but most commonly the area adjacent to the ileocecal valve. The inflammation in Crohn's disease is not necessarily confluent, frequently leaving "skip areas" of relatively normal mucosa. The Trans mural nature of the inflammation may lead to fibrosis and strictures or, alternatively, fistula formation.
[2] Ulcerative colitis (UC)
UC is an inflammatory disorder that affects the rectum and extends proximally to affect variable extent of the colon. Ulcerative Colitis nonspecific inflammatory bowel disease of unknown etiology that affects the mucosa of the colon and rectum. Ulcerative colitis is characterized by confluent mucosal inflammation of the colon starting at the anal verge and extending proximally for a variable extent [e.g., proctitis, left-sided colitis, or pan colitis].

Figure 2: Type of IBD [3]: (a) Crohn’s disease (b) Ulcerative Colitis
Other forms of IBD:
- Collagenous colitis
- Lymphocytic colitis
- Ischemic colitis
- Behçet’s disease
- Infective colitis
- Indeterminate colitis
MECHANISM OF INFLAMMATORY BOWEL DISEASE [4]

Figure 3: Mechanism of inflammatory bowel disease [5]
Crohn's disease and ulcerative colitis are chronic idiopathic inflammatory disorders of the GI tract; a summary of proposed pathogenic events and potential sites of therapeutic intervention. While Crohn's disease and ulcerative colitis share a number of gastrointestinal and extra intestinal manifestations and can respond to a similar array of drugs, emerging evidence suggests that they result from fundamentally distinct pathogenetic mechanisms. Histologically, the transmural lesions in Crohn's disease exhibit marked infiltration of lymphocytes and macrophages, granuloma formation, and sub mucosal fibrosis, whereas the superficial lesions in ulcerative colitis have lymphocytic and neutrophilic infiltrates. Within the diseased bowel in Crohn's disease, the cytokine profile includes increased levels of interleukin-12 (IL-12), interferon-g, and tumor necrosis factor-a (TNF-a), findings characteristic of T-helper 1 (TH1)-mediated inflammatory processes. In contrast, the inflammatory response in ulcerative colitis resembles more closely that mediated by the TH2 pathway.
[adsense:468x15:2204050025]
MESALAZINE [6-9]
Category: Anti-inflammatory agent, Non-steroidal anti-inflammatory agent
Chemical name: 5-Amino-2-Hydroxybenzoic acid[6]
Characteristics: appearsas off white to gray
Solubility: Slightly soluble in water, alcohol; more soluble in hot water; soluble in hydrochloric acid [6]
Melting point: 275-280 °C [7]
PKa value: pKa: 1.90, pKb: 5.43[7]
Molecular formula:C7H7NO3.
Molecular weight:153.14 g/mol [8]
The structural formula is shown below:

Figure 4: The chemical structure of Mesalazine
MECHANISM OF ACTION:
Although mesalamine is a salicylate, its therapeutic effect does not appear to be related to cyclooxygenase inhibition; indeed, traditional non-steroidal anti-inflammatory drugs actually may exacerbate IBD. Although the mechanism of action of mesalazine is not fully understood, it appears to be topical rather than systemic. Mucosal production of Arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalazine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon. Mesalamine appears to diminish inflammation by inhibiting cyclooxygenase and lipoxygenase, thereby decreasing the production of prostaglandins, and leukotrienes and hydroxyeicosatetraenoic acids (HETs) respectively. It is also believed acts as a scavenger of oxygen-derived free radicals, which are produced in greater in patients with inflammatory bowel disease.[9]
PHARMACOKINETIC[10]:
(Table 1)
|
Parameter |
Observation |
|
Bioavailability |
orally: 20-30% absorbed rectally: 10-35% |
|
Metabolism |
Rapidly & extensively metabolised intestinal mucosal wall and the liver |
|
Biological half-life |
5 hours after initial dose. At steady state 7 hours |
|
Excretion |
excreted mainly by the kidney as N-acetyl-5-aminosalicylic acid |
RIFAXIMIN [11-14]
Category: Gastrointestinal Agents, Anti-infective Agents
Chemical name: (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,36-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22,30-octamethyl-6,23-dioxo-8,37-dioxa-24,27,33-triazahexacyclo(23.10.1.14,7.05,³5.0²6,³4.0²7,³²)heptatriaconta1,3,5(35),9,19,21,25[36],26[34],28,30,32-undecaen-13-yl acetate[11]
Characteristics:Red Orange Crystalline Powder
Solubility: Soluble in DMSO (47 mg/ml), water (<1 mg/ml), ethanol (157 mg/ml), alcohols, and chloroform.[12]
Melting point: 218-227°C [13]
PKa value: pKa: 8.06, pKb: 4.42[13]
Molecular formula:C43H51N3O11.
Molecular weight:785.87g/mol [14]
The structural formula is shown below:
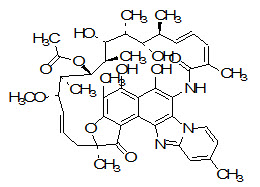
Figure 5: The structure of Rifaximin
MECHANISM OF ACTION [11]
Rifaximin is a semisynthetic, rifamycin-based non-systemic antibiotic, meaning that the drug will not pass the gastrointestinal wall into the circulation as is common for other types of orally administered antibiotics. It is used to treat diarrhea caused by E. coli.Rifaximin acts by inhibiting RNA synthesis in susceptible bacteria by binding to the beta-subunit of bacterial deoxyribonucleic acid (DNA)-dependent ribonucleic acid (RNA) polymerase enzyme. This results in the blockage of the translocation step that normally follows the formation of the first phosphodiester bond, which occurs in the transcription process.[13]
PHARMACOKINETICS [14]:
(Table 2)
|
Parameter |
Observation |
|
Bioavailability |
< 0.4% |
|
Metabolism |
Hepatic |
|
Biological half-life |
6 hours |
|
Excretion |
Fecal [97%] |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
COMBINATION THERAPY OF MESALAZINE AND RIFAXIMINX [15-18]
· Symptomatic uncomplicated diverticular disease using the combination Rifaximin/mesalazine followed by mesalazine alone.
· The effectiveness of the combination rifaximin/mesalazine followed by mesalazine alone to evaluate tolerability and effectiveness in symptomatic remission in uncomplicated diverticular disease.
· The results show that rifaximin/mesalazine followed by mesalazine alone is extremely effective in resolving symptoms in patients with symptomatic uncomplicated diverticular disease.
· Combination Rifaximin/mesalazine synergistic effect of these drugs: rifaximin should eliminate the micro flora (which seems to play a key role in determining both the symptoms and inflammation related to diverticular disease) and mesalazine should reduce the effect of the inflammatory cascade.
· Some studies have suggested that rifaximin [in combination with fiber or mesalazine] could be beneficial in the treatment and prevention of nonsevere, uncomplicated diverticular disease, effecting a better and faster relief of symptoms and a lower incidence of diverticulitis, recurrence, and rectal bleeding
· The combination of mesalazine and rifaximin was shown to be significantly more effective than rifaximin alone in preventing disease recurrence and improving symptoms in patients with symptomatic, uncomplicated diverticulitis and mild to moderate colonic obstruction.
· This complementary effect was probably mediated via the respective influences of rifaximin and mesalazine on the colonic microflora (which seems to play a key role in determining both disease related symptoms and diverticula inflammation) and the inflammatory cascade. Notably, in patients suffering from recurrent attacks of symptomatic, uncomplicated diverticular disease, continuous administration of mesalazine appeared to be more effective than cyclical administration in maintaining remission mesalazine appeared to be more effective than cyclical administration in maintaining remission.
· Combination patent:
Mesalamine and Rifaximin are two different types of drugs offering some symptomatic relief to the IBD patients. Mesalamine treats inflammation, whereas, Rifaximin reduces bio burden. However, in both cases, the disease is no completely cured and needs long term treatment and still the disease relapses.
· Rifaximin plus Mesalazine followed by Mesalazine is highly effective in resolving the signs and symptoms of symptomatic uncomplicated diverticular disease of the colon. Further studies are needed to demonstrate the effectiveness of Mesalazine in maintaining remission and preventing diverticulitis appearance during a longer follow-up.
MECHANISM OF ACTION OF COMBINED THERAPY

CONCLUSION
By reviewing the all literatures, the combination therapy was found to be effective in treatment of inflammatory bowel disease. This review represents individual pharmacology and pharmacokinetic of Mesalazine and Rifaximin as well as mechanism of action of combination of Mesalazine and Rifaximin in Inflammatory bowel disease.
This review will helpful for researcher in future studies and also for development of combined formulation of Mesalazine and Rifaximin as there no formulation is available.
REFERENCES
1. Inflammatory bowel disease Wikipedia the free encyclopedia, https://en.wikipedia.org/wiki/Inflammatory_bowel_disease 2/11
2. Diagram available form: www. nlm.nih.gov/medlineplus/ency/imagepages/19219.htm
3. Diagram available form: www. medicinenet.com/crohns_disease_pictures_slideshow/article.htm
4. Goodman & Gilman’s, The Pharmacological basis of therapeutics; - 11th Ed,
McGraw-Hill medical publishing division, 2006, 1047-1053
5. Diagram available form:
pubs.rsc.org/en/content/articlelanding/2010/fo/c0fo00103a/unauth
6. Mesalazine Drug Info.(database available on internet): Drug Bank Available form:
pubchem.ncbi.nlm.nih.gov/compound/5-Aminosalicylic%20Acid
7. Mesalazine Drug Info.(database available on internet)::
5-Aminosalicylic acid _ CAS 89-57-6 _ Santa Cruz Biotech
8. Mesalazine Drug Info.(database available on internet):Chemical book Available from:chemicalbook.com/productchemicalpropertiesCB1480481_EN.htm
9. Mesalazine Drug Info.(database available on internet): Drug Bank Available form:drug bank.ca/drugs/DB00244
10. Mesalazine Drug Info. (Database available on internet): Wikipedia. Available from:en. wikepedia.org/wiki/mesalazine
11. Rifaximin Drug Info.(database available on internet): Drug Bank Available form: drug bank.ca/drugs/DB01220
12. Rifaximin Drug Info.(database available on internet): Rifaximin _ CAS 80621-81-4 _ Santa Cruz Biotech
13. Rifaximin Drug Info.(database available on internet):Chemical book Available from:chemicalbook.com/productchemicalpropertiesCB5244184_EN.htm
14. Rifaximin Drug Info. (Database available on internet): Wikipedia. Available from:en. wikepedia.org/wiki/rifaximin
15. Giovanni Brandimarte, Antonio Tursi; Rifaximin plus mesalazine Followed by mesalazine alone is highly effective in obtaining remission of symptomatic uncomplicated diverticular disease; Med Sci Monit; 2004; 10(5); 70-73
16. Lupin Ltd, Harshal Jahagirdar, Umesh Badhe, Jisha Thomas, Rajesh Kulkarni, Shirishkumar Kulkarni et al; Therapeutic combinations and compositions for the treatment of gastrointestinal disorders; WO 2009047801 A1,2009
17. Tursi A., Papagrigoriadis S; Review article: the current and Evolving treatment of colonic diverticular disease; Alimentary Pharmacology & Therapeutics; 2009; 30(6); 532-546
18. Javier A. Herbert L; Rifaximin: A Novel Non absorbed Rifamycin for Gastrointestinal Disorders; Clin Infect Dis. 2006; 42(4); 541-547.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









