{ DOWNLOAD AS PDF }
ABOUT AUTHOR
AK MOHIUDDIN
Faculty of Pharmacy, World University of Bangladesh
Dhanmondi, Dhaka, Bangladesh
ABSTRACT
Pharmacoeconomics has been characterized as the depiction and examination of the cost of medication treatment to healthcare frameworks and society. All the more explicitly, pharmacoeconomic look into is the way toward recognizing, estimating, and contrasting the costs, dangers, and advantages of programs, services, or treatments and figuring out which elective delivers the best wellbeing result for the asset contributed. This data can help clinical chiefs in picking the most cost-effective treatment alternatives. Pharmacoeconomics is a division of results examine that can be utilized to measure the estimation of pharmaceutical care items and services. Pharmaceutical care has been characterized as the mindful arrangement of medication treatment for the reasons for accomplishing unequivocal results.

Figure 1. Graphical Extract
Materials and Methods:
Research conducted a year-round comprehensive literature search, which included technical newsletters, newspapers journals, and many other sources. The present study was started from the beginning of 2018. PubMed, ALTAVISTA, Embase, Scopus, Web of Science, and the Cochrane Central Register of was thoroughly searched. The keywords were used to search for different publishers’ journals like Elsevier, Springer, Willey Online Library, Wolters Kluwer were extensively followed. Medicine and technical experts, pharma company representatives, hospital nurses and chemists were given their valuable suggestions. Projections were based on estimates of drug and therapy related cost, cost of being ill and hospitalization and cost of well-being. Pharmacists role in allied areas of cost calculation and minimizing through ADR management, prevent disease and hospitalization and drug selection were given the highest priority.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2648
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 7, Issue 03 Received On: 03/01/2019; Accepted On: 29/01/2019; Published On: 01/03/2019 How to cite this article: Mohiuddin, A.K. 2019. Pharmaco-Economics: The Cost of Health. PharmaTutor. 7, 3 (Mar. 2019), 1-18. DOI:https://doi.org/10.29161/PT.v7.i3.2019.1 |
INTRODUCTION
Globally patients are affected by the high price of medicines. In many developing countries a high percentage of total health expenditure is financed by household out of–pocket expense. Many poor people frequently face a bitter decision between purchasing medication or buying such necessities as grocery and clothing due to limited resources and the high price of the prescription. Therefore, medication and drug therapy are an important matter to the society mainly those in need of medical services with limited financial resources. Human services costs have been expanding every year more than the normal rate of expansion. This proceeded with increment in costs has brought about a need to see how restricted assets can be utilized most productively and effectively. Pharmacoeconomics role in many developing countries is in early stages with a limited knowledge of the subject matter. Therefore, lack of education and understanding of the topic is limiting the decision making by the health providers and health authorities.

Statement of the problem
Pharmacoeconomics (PE) role in many developing countries is in early stages with a limited knowledge of the subject matter. Therefore, lack of education and understanding of the topic is limiting the decision making by the health providers and health authorities. Thereby, creating incapability in developing a plan for purchasing the maximum amount of benefits for a given resource use. Accordingly, unable to assist clinicians in choosing the most affordable options. Pharmacoeconomics and outcome research have a very significant function in medication expenditure management. Subsequently, healthcare moderateness has turned into a noteworthy issue in the lives of society and their prosperity. For this reason, understanding PE and the importance of its applications are vital in reducing healthcare wastage as explicit methodology, education, and initiative required for its progress.
The General Necessity of Pharmacoeconomics
U.S. NHEA estimated health care spending grew 4% in 2017, reaching USD 3.5 trillion or USD 10,739 per person. As a share of the nation's GDP, health spending accounted for 18% (Web CMS). About 12% (over USD 900 per person) of health care expenditures were for medications in 2010. Health care costs have been increasing each year more than the average rate of inflation. CMC further realized aggregate healthcare spending would grow at a 5.8% average annual rate from 2015 to 2025, or 1.3% higher than the expected annual increase in the gross domestic product. This is causing more interest in new strategies across employers for drugs or administration. Increasing healthcare costs have forced employers to reassess the healthcare benefits they offer to employees (Vogenberg et.al., 2018). This continued increase in costs has resulted in a need to understand how limited resources can be used most efficiently and effectively. Pharmacoeconomics is essential in several sectors such as
1. Healthcare industry in order to decide amongst precise research and development options.
2. Second, it is needed in government to determine program benefits and its operating expense.
3. The third area of need is in the private sector to facilitate the formulation of insurance benefits coverage. Basically, the PE is needful in following manner;
• In Industry, it is useful in deciding among specific research and development alternatives.
• In Government- Determining program benefits and prices paid and in Private Sector it can be used for designing insurance benefit coverage.
4. Additionally, it describes the economic relationship involving drug research, drug production, distribution, storage, pricing and its use by the society. It runs on the thread of our socioeconomic system, which regulates and influences all the sectors involved in pharmaceuticals (Gattani. Et.al., 2009).
Perspectives of Pharmaco-economics
• Patient Perspective--Patient perspective is paramount because patients are the ultimate consumers of healthcare services. Costs from the viewpoint of patients are basically what patients pay for an item or administration—that is, the segment not secured by protection.
• Provider Perspective--Costs from the provider's perspective are the actual expense of providing a product or service, regardless of what the provider charges. Providers can be hospitals, MCOs, or private-practice physicians. From this perspective, direct costs such as drugs, hospitalization, laboratory tests, supplies, and salaries of healthcare professionals can be identified, measured, and compared.
• Payer Perspective--Payers include insurance companies, employers, or the government. From this perspective, costs represent the charges for healthcare products and services allowed or reimbursed by the payer. The primary cost for a payer is of a direct nature. However, indirect costs, such as lost workdays (absenteeism), being at work but not feeling well and therefore having lower productivity (presenteeism), also can contribute to the total cost of healthcare to the payer.
• Societal Perspective--Theoretically, all direct and indirect costs are included in an economic evaluation performed from a societal perspective. Costs from this perspective include patient morbidity and mortality and the overall costs of giving and receiving medical care. An evaluation from this perspective also would include all the important consequences an individual could experience. In countries with nationalized medicine, society is the predominant perspective (TRASK, 2011).
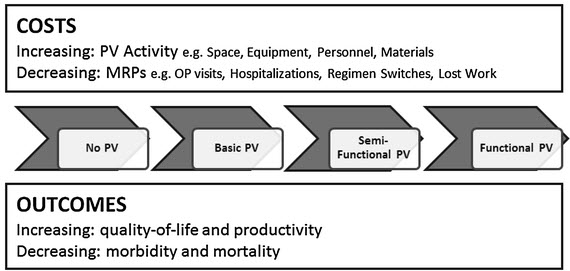
Figure 2. Framework for assessing the economic value of pharmacovigilance in low- and middle-income countries.
Countries were classified into four levels. In level 1 (no PV), there are no legal or structural frameworks, no coordinated surveillance activities, and PV activities are not coordinated nationally. In level 2 (basic PV), policy and legal frameworks exist, institutions, guidelines, and procedures exist, and stakeholder roles are recognized albeit poorly coordinated. Additionally, the AE reporting system does not cover all sources of MRPs, signal generation and risk evaluation are poor, and the system lacks active signal evaluation and risk management. In level 3 (semi-functional PV), structural and organizational frameworks exist to collect and collate safety data and evaluate risks and benefits by passive and active surveillance. However, the countries lack risk management, risk prevention, and risk communication capacity. In level 4 (functional PV), a PV structure exists that permits passive and active surveillance, risk evaluation, risk communication, and regulatory action.
Pharmacoeconomic Doctrine
• Pharmacoeconomics search to define and analyze the costs of medication therapy to the healthcare system and society (Power et.al., 2014)
• Principles employed on different ECHO (economic, humanistic, and clinical outcomes) using the methodology of PE (Kozma et.al., 1993; Telser et.al., 2011)
• Health care costs categorized as direct medical, direct nonmedical, in direct nonmedical, intangible, and opportunity costs (Babigumira et.al., 2014)
• In comparing various health care choices, economic valuation methods used, including cost minimization, cost-benefit, cost-effectiveness, and cost-utility analyzes (Jo et.al., 2014)
• Comparisons expressed in monetary units, ratios, or mixed units nine such as dollars per quality - adjusted life-year) (SIAPS and USAID, 2017).
• The cost of illness assessments classifies and estimates the inclusive cost of a particular illness for a distinct population. Nevertheless, COI is not utilized to relate alternative choices (Waning et.al., 2001)
• In pharmacy practice, Pharmacoeconomic methods employed for effective management of formulary, treatment of individual patient, termination of medication program, and resource distribution (Surji, 2015)
Several factors should be measured when evaluating published pharmacoeconomic studies. Such factors are
a) Research objective
b) Education perspective
c) Pharmacoeconomic method
d) Study design
e) Choice of interventions
f) Costs and consequences
g) Discounting
h) Study results
i) Sensitivity analysis
j) Research conclusions
k) Sponsorship (Dubois, 2010)
Pharmacoeconomic challenges
The major challenges for Pharmacoeconomics are to
• Develop a guidelines and procedure for standards of practice.
• Build a framework for well-skilled producers and clients of PE evaluations.
• Continuing education on the pertinent features of this discipline for practitioners, government
• officials, private sector executives.
• Stable funding to support applied pharmacoeconomic research
• Creating a cadre of trained producers and consumers of pharmacoeconomic work.
• Lack of full appreciation of the potential importance and application of Pharmacoeconomics studies.
• Poor technical skills of healthcare professionals, especially of pharmacists.
• Lack of appropriate database of the healthcare system in order to bring about research adaptation from another country (Ambrosioni, 2001; Chabot, 2008; et.al., 2008; Catić et.al., 2013; Milne, 1994)
Scope of Pharmacoeconomics
1) To Pharmaceutical manufacturers: Pharmacoeconomics can be a very useful tool long before a drug is approved for use by the FDA. Pharmaceutical manufactures need to spend enormous resources in the drug development process. If proper pharmacoeconomic research is conducted the manufactures can avoid spending vast resources to the development of a drug that does not provide competitive advantage. Competitive advantage in the present healthcare environment may be defined as a drug that is cost effective. Cost effective can mean a drug that is
• Less costly and at least as effective as an alternative;
• More effective and costlier than an alternative, but improved health outcomes justify additional expenditures, or
• Less effective and less costly than an existing alternative, but a viable alternative for some patients.
• Cost efficacy and QOL components can be incorporated into appropriate phase III studies to provide additional information regarding a drugs impact on patient outcome.
If such parameters are applied systematically to all new treatment candidates, the scientific basis of drug therapy decision making will increase substantially.

Figure 3. Data Flow Diagram Clinical Trial Data Management (CDM) Plan (Source: crossfithartford.com)
CDM is the process of collection, cleaning, and management of subject data in compliance with regulatory standards. The primary objective of CDM processes is to provide high-quality data by keeping the number of errors and missing data as low as possible and gather maximum data for analysis. If proper pharmacoeconomic research is conducted the manufactures can avoid spending vast resources to the development of a drug that does not provide competitive advantage (Krishnankutty B, Bellary S, Kumar NB, Moodahadu LS. Data management in clinical research: An overview. Indian J Pharmacol. 2012;44(2):168-72).
2) To Healthcare Practitioners: One of the primary uses of PE in clinical practice is to aid clinical and policy decision making. Complete pharmacotherapy decisions should contain three basis evaluation components;
* Clinical
* Economic
* Humanistic outcomes
• Through the appropriate application of Pharmacoeconomic principles and methods incorporating these three critical components into clinical decision can be accomplished. Pharmacoeconomic data can be a powerful tool which supports various clinic decisions, including
* effective formulary management,
* individual patient treatment,
* medication policy, and resource allocation.
• Pharmacoeconomic data can support the inclusion or exclusion of a drug on or from the formulary and support practice guidelines that promote the most cost-effective or appropriate utilization of pharmaceutical products. Various strategies can be used to incorporate PE into formulary decision making.
• In fact, the pharmacoeconomic assessment of formulary action is becoming a standardized part of many pharmacy and therapeutic (P&T) committee decision making process, when competing for hospital resources, PE can provide the data necessary that a pharmacy service maximizes the resources allocated to it by hospital administration.
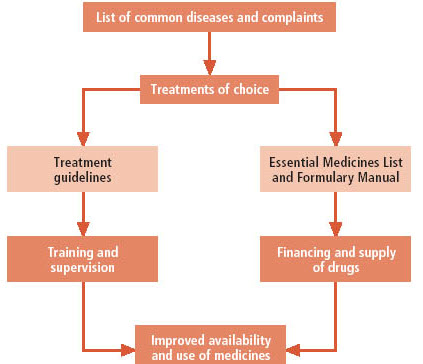
Figure 4. the relationship between STGs and EMLs and how these affects respectively the use and the availability of medicines (Source: Web WHO. Drug and Therapeutics Committees - A Practical Guide).
Strict adherence to a formulary list alone will not improve treatment practice if drug selection is not based on STGs (i.e. if there is no consistency between the formulary list and the STGs). Furthermore, essential medicines can also be used inappropriately if there are no guidelines for disease management. Ideally, a formulary list should be developed after the appropriate treatment guidelines for common diseases have been identified or developed. In many countries, there are already national STGs and other texts on standard treatment protocols that can be followed and used as a starting point when developing a hospital formulary list or local STGs. Once a formulary list is established, a formulary manual, containing information on all the medicines in the formulary list, can be developed.
3) To Pharmacists:
Drug use evaluation is one of the important services provided by pharmacists. Ideally, that value should be translated into patient and financial outcomes. Apart from concentrating on inappropriately prescribed therapy and overprescribing, drug use evaluation focuses on the most cost-effective therapy. A high degree of sophistication is required in order to make such determination fairly, considering patient factors, disease factors, and other issues. Drug formulary services, Pharmacy and therapeutics committees are viewed as a means of reducing drug budgets and have had some value in encouraging drug therapy cost considerations, but they do not provide incentives to take into account overall medical costs, nor do they necessarily consider all consequences such as potential drug interactions, adverse reactions, and treatment response rates. Conducting cost-effectiveness studies allows an evaluation of total costs and consequences from various perspectives (Jo, 2014; Scaria et.al., 2015; Ahmad et.al., 2013; Trask, 2011; McGhan et.al., 1978)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Application of Pharmacoeconomics
PE utilizes for inform decision-making; moreover. This decision making in pharmacy perspective divided into two fundamental points:
• Evaluation of drug therapy
• Evaluation of clinical pharmacy.
Traditionally, PE methods were applied in the field of hospital pharmacy activities. The cost-effectiveness data were used to support the addition or deletion of a drug to or from a hospital formulary. However, currently, the pharmacoeconomic measurement of formulary procedures became a standardized part of numerous pharmacies and therapeutic team. In the past PE was mostly applied to drug therapy evaluations; however, different studies reveal a shift over the years in using PE for the justification of pharmacy services decision-making. Possible barriers to application of PE for drug decision-making identified as follows:
• The inadequacy of PE sophistication by hospital administrators and pharmacy directors.
• Incompetency of PE sophistication by pharmacy practitioners that create and interpret PE data.
• Deficiency of organizational resources in the application of PE such as time and financials.
• Financial plan and budgetary responsibilities (Web Pharmadost, 2017).
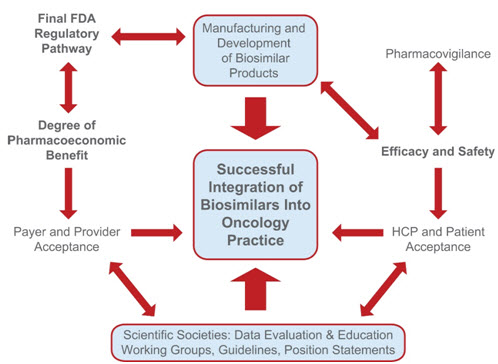
Figure 5. Parameters influencing the successful uptake and integration of biosimilars into US oncology practices.
The US FDA will provide a finalized pathway for biosimilar approval; this pathway will, in turn, influence the manufacturing and development process and the amount of clinical data needed for approval. The efficacy and safety of biosimilars will be monitored via ongoing pharmacovigilance practices to ensure that potential immunogenicity or adverse events with a given biosimilar can be identified quickly and addressed. Biosimilars have the potential to offer cost-savings with comparable efficacy and safety to innovator products. They are being used in the European Union, Canada, Japan, and Australia and may help with improving health outcomes while minimizing costs to patients and global healthcare systems. The overall value of a biosimilar is not determined solely by its pricing. Efficacy and safety relative to the reference biologic drug and competitive agents as well as development and manufacturing costs, treatment administration costs, and results from long-term safety monitoring are considered (Source: Henry et.al., 2014).
Pharmacoeconomic studies find value in
• Fixing the price of a new drug and re-fixing the price of an existing drug
• Finalizing a drug formulary
• Creating data for promotional materials of medicines.
• Compliance of requirement for drug license.
• Including a drug in the medical/insurance reimbursement schemes.
• Introduction of new schemes and programs in hospital pharmacy and clinical pharmacy.
• Drug development and clinical trials (National Library of Canada, 1997).
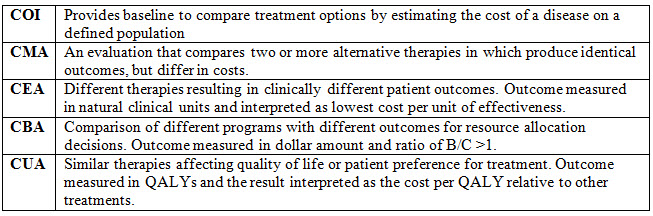
| Exhibit 2. Primary Components of Pharmacoeconomic Studies |
| The two primary components of pharmacoeconomic studies are measures of costs and outcomes. It can be measured using the five methods such as, Cost minimization analysis (CMA), Cost of Illness (COI), Cost effectiveness analysis (CEA), Cost-utility analysis (CUA), and Cost-benefit analysis (CBA). |
Pharmacoeconomic Components
A. Cost of drug
Cost is defined as the value of the resources consumed a drug therapy of interest. It is the amount paid to the suppliers by the patient. Consequence is defined as the effects, outputs, or outcomes of the program of drug therapy of interest.
• Direct medical cost : This is what is paid for specialized health resources and services. It includes the physician’s salaries; the acquisition cost of medicine; consumables associated with drug administration; staff time in preparation and administration of medicines; laboratory costs of monitoring for effectiveness and adverse drug reactions.
• Direct non-medical cost : This includes cost necessary to enable an individual receive medical care such as lodging, special diet and transportation; lost work time (important to employers) such as acute Otitis media in pediatric patients with professional parents who lost work time during the treatment of their kid.
• Indirect cost : This is the cost incurred by the patient, family, friends or society. Many of these are difficult to measure, but should be of concern to society as a whole. This includes productivity loss in the society; unpaid care givers; lost wages; expenses of illness borne by patients, relatives, friends, employers and the government and; loss of leisure time.
• Intangible costs: These are costs related with the patient’s pain and suffering; worry and other distress of the family members of a patient; effect on quality of life and health perceptions. For example, patients of rheumatoid arthritis, cancer or having terminal illnesses in which quality of life is suffered due to adverse reactions of the drug treatment. These are difficult to measure in monetary terms but represent a considerable concern for both doctors and patients. Quality adjusted life year (QALY) is one method by which intangible costs can be effectively integrated in PE analysis.
The cost can be measured in following ways:
• Cost / Unit
• Cost / Treatment
• Cost / Person
• Cost / Person / Year
• Cost / Case Prevented
• Cost / Life Saved
• Cost / Daly (Disability-Adjusted Life Year)
B. Outcomes (The second fundamental component of a pharmacoeconomic study is outcomes or benefits). The expected benefits might be measured in:
• “Natural” units e.g. years of life saved, strokes prevented, and peptic ulcers healed etc.
• “Utility” units - Utility is an economist’s word for satisfaction, or sense of well-being, and is an attempt to evaluate the quality of a state of health, and not just its quantity. Utility estimates can be obtained through direct measurement (using techniques such as time trade off or standard gambles, or by imputing them from the literature or expert opinion. They are often informed by measures of quality of life in different disease states (Gattani et.al., 2009; TRASK, 2011; Ambrosioni, 2001; Jo, 2014)
Methods of Pharmacoeconomics
Pharmacoeconomics evaluation compares economic, clinical, and humanistic outcomes associated with different therapies. The evaluation mechanisms as described are frequently helpful in representing the cost impact of innovative treatments, yielding superior acceptance by healthcare providers, administrators, and the public.
The pharmacoeconomic methods divided into two separate classes:
1. An economic evaluation, such as Cost of Illness, Cost Benefit, Cost Effectiveness, Cost
Minimization, and Cost Utility.
2. Humanistic evaluation similar to Quality of life, Patient preferences, Patient satisfaction. These techniques used in a variety of fields and are applied increasingly to healthcare and Pharmaceuticals.
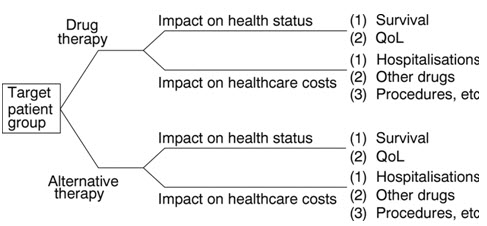
Figure 6: Drug Therapy Vs Alternative Therapy, Nature of Pharmacoeconomic Assessment (Source: Drummond, 2006). As significant progress was made in recent years and there are many alternative treatments, which are indicated according to the stage and the type of the disease, the age and health status of patient, and vary from surgery to hormonal treatment and chemotherapy. Time horizon, stage of the disease, patient age, therapy onset, benefit duration and time to recurrence may influence the results. Pharmacoeconomic analyses of alternative therapy options will improve decision-making and will help to optimize the use of scarce health care resources allocated to the patient care. Costs involved in pharmacoeconomic evaluation can be mainly divided into financial cost (mandatory cost) and economic cost (resource for which no mandatory payment is made) opportunity cost is the benefit foregone when selecting one therapy alternative over the next best alternative. Several costs can be measured when weighing up the cost of any invention. The first step in any cost analysis is identification of the various costs. These can be direct, indirect and intangible.
Economic evaluation methods
A. Cost-of-Illness Evaluation
A cost-of-illness (COI) evaluation identifies and estimates the overall cost of a particular disease for a defined population. This evaluation method is often referred to as burden of illness and involves measuring the direct and indirect costs attributable to a specific disease. The costs of various diseases, including diabetes, mental disorders, and cancer, in the United States have been estimated. By successfully identifying the direct and indirect costs of an illness, one can determine the relative value of a treatment or prevention strategy. For example, by determining the cost of a particular disease to society, the cost of a prevention strategy could be subtracted from this to yield the benefit of implementing this strategy nationwide. COI evaluation is not used to compare competing treatment alternatives but to provide an estimation of the financial burden of a disease. Thus, the value of prevention and treatment strategies can be measured against this illness cost.
B. Cost-Minimization Analysis
Cost-minimization analysis (CMA) involves the determination of the least costly alternative when comparing two or more treatment alternatives. With CMA, the alternatives must have an assumed or demonstrated equivalency in safety and efficacy (i.e., the two alternatives must be equivalent therapeutically). Once this equivalency in outcome is confirmed, the costs can be identified, measured, and compared in monetary units (dollars). CMA is a relatively straightforward and simple method for comparing competing programs or treatment alternatives as long as the therapeutic equivalence of the alternatives being compared has been established. If no evidence exists to support this, then a more comprehensive method such as cost-effectiveness analysis should be employed. Remember, CMA shows only a “cost savings” of one program or treatment over another. Employing CMA is appropriate when comparing two or more therapeutically equivalent agents or alternate dosing regimens of the same agent. This method has been used frequently, and its application could expand given the increasing number of “me too” products and generic competition in the pharmaceutical market.
C. Cost-Benefit Analysis
Cost-benefit analysis (CBA) is a method that allows for the identification, measurement, and comparison of the benefits and costs of a program or treatment alternative. Both the costs and the benefits are measured and converted into equivalent dollars in the year in which they will occur.
The CBA is also defined as a systematic process for calculating and comparing benefits and costs of a project, decision or government policy (hereafter, "project"). Broadly, CBA has two purposes:
1. To determine if it is a sound investment/decision (justification/feasibility),
2. To provide a basis for comparing projects. It involves comparing the total expected cost of each option against the total expected benefits, to see whether the benefits outweigh the costs, and by how much.
CBA is related to, but distinct from cost-effectiveness analysis. In CBA, benefits and costs are expressed in monetary terms, and are adjusted for the time value of money, so that all flows of benefits and flows of project costs over time (which tend to occur at different points in time) are expressed on a common basis in terms of their “net present value.”
Table 1: CBA interpretation ratio
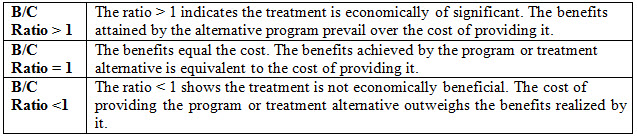
CBA engaged when
• Costs and benefits of desired therapeutic choices do not occur concurrently.
• Paralleling treatment plans with different objectives, because all benefits changed into dollar units.
• Evaluating a particular program or relating multiple programs. Nevertheless, treasuring health benefits in monetary terms can be confusing and controversial.
D. Cost-Utility Analysis
Cost-utility analysis (CUA) is an additional method for comparing treatment options. CUA incorporated patient preferences and health-related quality of life (QoL). The use of CUA is the most suitable method to utilize when comparing treatment alternatives that are life extending with serious adverse effects. For instance, treatment of cancer with chemotherapy, as well as those which produce a reduction in morbidity rather than mortality as treatment of arthritis. Cost measured in dollars. The term ‘quality-adjusted life year’ (QALY) is a standard measure of health status used in CUA combining morbidity and mortality data. The number of QALYs lived by an individual in one year is simply:
QALYs lived in one year = 1 * Q with Q ≤ 1
where Q is the health-related quality of life weight attached to the relevant year of life. The chosen treatment alternative is that with the lowest cost per QALY. Thus, QoL is the most important health outcome being examined as per patient preferences
E. Cost-Utility Analysis
CEA involves comparing therapeutic programs or treatment alternatives with different safety and
Efficacy profiles. It is an approach used for identifying, measuring, and comparing the significant costs and consequences of alternative interventions. A value measured in dollars, and outcomes measured in terms of obtaining a particular therapeutic outcome. These results often expressed in physical units, natural units, or non-dollar units for example lives saved, cases cured, life expectancy, or mm Hg drop in blood pressur. The outcome of CEA expressed as a ratio as well. The two possible methods for the CEA quotient are an incremental cost-effectiveness ratio (ICER) and an average cost-effectiveness ratio (ACER).
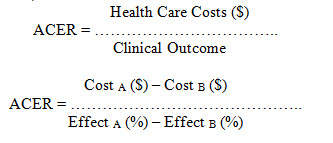
CEA is mainly practical in balancing cost with patient outcome, determining which treatment alternatives represent the best health outcome per dollar spent. It is also valuable in indicating when it is appropriate to measure outcome in terms of obtaining a particular therapeutic intention. For example, in comparing antiemetic agent for improvement of guidelines for the deterrence of emesis induces by chemotherapy, the Cost-effective analysis implemented (Gattani et.al., 2009; TRASK, 2011; Scaria et.al., 2015; Ahmed et.al., 2013; McGhan et.al., 1978; National Library of Canada, 1997)
Table 2: Summary of PE Methods.
Pharmacoeconomic Evaluation Steps
Pharmacoeconomic evaluation process contained several necessary steps useful in the health care system and nearly any therapeutic area or healthcare service.
1) Define the problem: A general question might be, "which antiemetic regimen represents the best value for the prevention of chemotherapy-induced emesis (CIE)?" However, a more succinct and measurable the problem would be "which regimen is the best value for preventing acute CIE patients receiving highly emetogenic chemotherapy?"
2) Gather various functional team members: Team members differ contingent on the analysis but may include members from medicine, nursing, pharmacy, hospital administration, and information technology and systems.
3) Define the perspective: Choose a study perception typically pertinent to the problem. For example, if the problem is similar as given in Step 1, then the institution or health care system perspective is a most appropriate chosen alternative.
4) Identify therapeutic preferences and outcomes: Treatment options include pharmacologic and non-pharmacologic choices. However, it should comprise all clinically relevant options. The identified outcomes should consist of both positive and negative clinical outcomes.
5) Determine the applicable pharmacoeconomic method: The methods of Pharmacoeconomic to choose from are CMA, CBA, CEA, and CUA. Employing the incorrect method can adversely affect medication decisions influencing both costs Moreover, quality of care.
6) Set a financial value on treatment alternatives and outcomes: Employing a financial value on treatment options and outcomes for drug administration and cost of purchase as well as the cost of positive and negative medical results.
7) Pinpoint resources to implement evaluation in a proficient method: Depending on the study, the necessary resources will fluctuate. However, it possibly will comprise entrance to medical or computerized records, regular medical personnel salaries specifically medical staff.
8) Detect possibilities that outcomes can transpire in the population under study: What are the likelihoods of the results recognized in step 4 (e.g. identifying therapeutic preferences and outcome) in fact to occur practically? By means of principal literature and professional selection, these probabilities can be acquired and perhaps expressed by way of efficacy rates and occurrence of ADRs.
9) Implement decision analysis: Numerous economic evaluations can be conducted by utilizing decision analysis, including CEA. Even though, Decision analysis and decision tree may not require forever evaluations in PE. However, they provide firm support for the verdict at hand. Using a decision tree, treatment options, results, and probabilities presented explicitly. Additionally, it can reduce algebraically to a single value for comparison (i.e., CEA ratio).
10) Discount costs, sensitivity, incremental cost analysis: Prospecting costs and consequences discounted back to their present value. Furthermore, sensitive variables tested over a clinically related range, and outcomes recalculated. If applicable, an incremental analysis of the costs and consequences should be made.
11) Present the outcome of study: The study results presented to the cross-functional team and the proper committees.
12) Cultivate a policy and procedure for intervention: Exploit the study outcomes and develop a policy and an intervention for the enhancement of healthcare quality and maintain efficiency.
13) Implement policy and educate professionals: Devote sufficient time and resources are cleverly executing the policy or intervention. Moreover, the healthcare professionals affected by the policy must be educated using different strategies such as verbal, written and online communication technology.
14) Follow-up documentation: Monitoring collected data after the implementation of policy and intervention for a practical period as this information will offer a response to the achievement and quality of the policy or intervention (Ahmed et.al., 2013; National Library of Canada, 1997; Parkis, 2006)
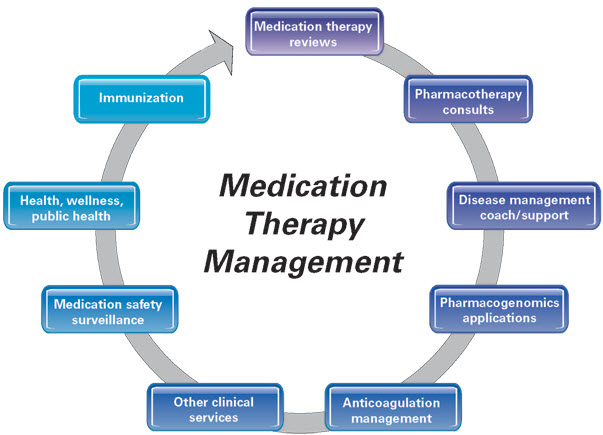
Figure 7. Medication Therapy Management Services (Source: APhA, 2008). Pharmacotherapy consult incorporates the pharmacist’s expertise into achieving desired therapeutic goals for patients by promoting safe, appropriate, and cost-effective use of medications. Patients requiring pharmacotherapy consults may have a single or multiple complex medical condition that require medication therapy to effectively manage. Pharmacists providing these services typically have advanced expertise and training in the subject area and may be Board Certified in their specialty by the Board of Pharmaceutical Specialties.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Pharmacoeconomics – A Tool for Pharmacists
PE helps us to make decisions about the use of medicines. Most pharmacoeconomic studies in health care are cost-effectiveness studies set out to demonstrate how to achieve an objective with the least use of resources. This should not be confused with efficiency, which measures how well we use resources in order to obtain the desired outcome.
• PE is used at all stages in the development of medicines by the pharmaceutical industry, when medicines are researched, produced and marketed. Some countries insist on pharmacoeconomic evaluations as part of the licensing process. Most hospital pharmacists use PE to assist with making decisions involving formularies and how medicines can be used in a more cost-effective or cost beneficial manner. Knowledge of health economics coupled with political insight is essential to understand resource allocation and expenditure in a modern health care system. Pharmacists, with their unique knowledge of medicine, are crucial in using pharmacoeconomic analysis to influence expenditure and distribution of resources on medicines.
• The basis of financing secondary care is currently changing. Under “payment by results”, providers of care are paid for each patient spell according to a national tariff, which is based on a national average cost for a particular patient spell. As foundation trusts increase, the number of hospitals that depend on tariff payments for their income also grows. Therefore, using the most efficient methods of working to reduce cost and maximize benefits is becoming increasingly important.
• PE is part of the tool bag pharmacists can use to improve the efficiency of their hospital. In theory, if hospitals improve their efficiency and deliver increased activity the trust will make a profit, which should then be invested in improving health care. In some medical disciplines the medicines element to the overall tariff price can be considerable, and savings on costs of medicines can make the difference between a profit and loss for the trust.
• The application of PE to improve the efficient use of medicines is a key component in this productivity drive. Although the clinical role of the profession is appreciated, it is the role of the pharmacist in advising on medicines expenditure and ensuring economical use of medicines that has increased demand for their services.
• In many directorates the only person with the required knowledge, experience and expertise to manage the medicines budget is the directorate pharmacist. Medicines management technicians are now also seen as essential to the overall improvement in efficiency and reduction on Medicines expenditure. Knowledge of health economics and application of its techniques is essential to today’s pharmacist (Gyllensten et.al., 2014; Beijer et.al., 2002; Leendertse et.al., 2008; Sawyer et.al., 2016)
Pharmacists in Healthcare Cost Minimization
The role of the pharmacist has evolved substantially in recent decades. The traditional activities of the profession primarily focused on the dispensing and supply of medications, while interaction with other healthcare professionals was somewhat limited.
• Cost savings and Avoidance: Cost savings allude to reductions in current spending due to changes in the expense on a patient’s treatment, e.g. switching from intravenous to oral therapy where appropriate. In contrast, cost avoidance refers to an intervention that reduces potential future spending that may have occurred without the intervention. With their unique knowledge of medicines, pharmacists are central figures in decreasing healthcare expenditure through cost savings on medicines and cost avoidance. It has been estimated that 5–6% of all hospitalizations are drug-related Patients affected by an ADE. and the additional costs of patients experiencing ADEs have been estimated to USD 2284–5640 per patient. Medication errors are very costly to healthcare systems, but a large portion of these are preventable (Yach et.al., 2004; Tinetti, 2012)
• Chronic disease management: Chronic diseases are the leading cause of death and disability worldwide, and their management accounts for more than two-thirds of global healthcare expenditure (Bunting et.al., 2008; Khdour et.al., 2009)Pharmacists in primary care have the skills to manage patients with long-term conditions, and this can result in both clinical and cost benefits for a variety of chronic illnesses, such as cardiovascular disease, chronic obstructive pulmonary disease, and diabetes. When compared with usual medical care, one study found that pharmacist-run services made savings of USD 647,024 by preventing hospital admissions and emergency department (ED) visits (Morello et.al., 2006; Hall et.al., 2011; Cutler, 2018)
• Prevention of Non-adherence Related Hospitalization: Annual costings of medication non-adherence range from USD100 to USD 290 billion in the USA, euro 1.25 billion in Europe and approximately USD A7 billion in Australia. Additionally, 10% of hospitalizations in older adults are attributed to medication non-adherence with the typical non-adherent patient requiring three extra medical visits per year, leading to USD 2000 increased treatment costs per annum. In diabetes, the estimated costs savings associated with improving medication non-adherence range from USD 661 million to USD 1.16 billion (Rotta et.al., 2015; Lee et.al., 2006). Studies have shown that pharmacists can improve medication adherence rates, resulting in improved patient outcomes. 20%- 30% of dollars spent in the US health care system have been identified as wasteful. Pharmacists are key players in the pharmaceutical supply chain and are in a position to contribute to the reduction of medication waste. Pharmacists may also assist patients by recommending lower cost brands (Web CMS, 2011; Web Henry J Kaiser Family Foundation, 2012; Bekker et.al., 2018; Usherwood, 2017; Annual Report Of The Boards Of Trustees, 2012)
• Facing the Cost Escalation: Projections indicate health care will account for 20% of the US gross domestic product by 2020 (IMS Institute for Healthcare Informatics, 2015; Wheeler et.al., 2017). And global medicine use with both prescription and over-the-counter (OTC) medicines is increasing, and estimated to reach 4.5 trillion doses in 2020; an increase of 24% from 2015 (Bettington et.al., 2018). As the global population ages, healthcare organizations are challenged with the increasing burden of chronic diseases and polypharmacy among older adults. Pharmacists have a major role in lowering costs by critically reviewing the pharmacotherapy of multimorbid elderly patients. The reduction of inappropriately prescribed medicines not only produces savings in the cost of each individual medicine but also reduces the risk of ADEs that often contribute to prolonged and expensive hospital admissions.
• Reduce burden of Medication Return/ Disposal: The accumulation of unwanted medicines at home can result in accidental ingestion, or lead to confusion, and out-of-date medicines can become toxic or ineffective. Adverse consequences of inappropriate disposal of medicines in landfill and via the sewerage system have been reported, including identity theft from personal information on medicine labels disposed in garbage, and concentrations of medicines detectable in surface and drinking water. The Return Unwanted Medicines Project collected over 704 tons of unwanted medicines in 2016. This included prescription medicines, dose administration aids (which may include multiple medicines), over-the-counter medicines and complementary medicines (Bettington et.al., 2018). The majority of returned medications contained greater than 75% of the original amount issued. Identification of therapeutic groups having higher rates of returns due to medication changes or surplus to requirements. MURs was made available in through the NHS in 2005 and are free to patients, which ensure patients take medicines as prescribed, reduce waste, return and cost involved.
• OTC Selection and Management of Minor Ailments: The number of medicines available without a prescription is growing rapidly; there are currently over 300,000 OTC medicinal products available in the US market alone. Self-medication with OTC products has been shown to contribute to ADRs and hospital admissions. With the advice and recommendations of a community pharmacist, patients can avoid spending money on ineffective or potentially harmful OTC medications; this helps limit further healthcare utilization by patients, such as GP or ED visits. This characteristic feature of community pharmacies provides a platform for more proactive contribution in self-care and managing a range of minor ailments, one of the enhanced population health services to be provided by community pharmacy professionals in UK ( James et.al., 2009; Latif, 2018; NHS, 2005)
• Potential of Hospital Pharmacists: In Africa and Asia, hospital pharmacists have been predominantly limited to dispensary-based roles, meaning that their expertise in medicine management is being underutilized. In many countries, hospital pharmacists have expanded their roles beyond the dispensary, and now routinely provide clinical pharmacy services at ward level, which includes reviewing patients’ medications and advising other healthcare professionals with regard to pharmacotherapy (Ayele et.al., 2018). However, many studies have proven that pharmacist interventions have a positive impact on hospital budgets, but it is difficult to elucidate which interventions were the most cost-effective. Cost-saving interventions often include discontinuing unnecessary medicines, switching to less expensive agents, or altering the route of administration. Several review articles have demonstrated the overall cost-effectiveness of hospital pharmacists’ activities in a wide variety of clinical specialties, such as ED and intensive care unit (ICU) pharmacists, pharmacists managing therapeutic drug monitoring of antibiotics and antiepileptics, and those who specialize in the optimization of antimicrobial therapy (Auta et.al., 2015)
• Medicines Reconciliation and Transitions of Care: An accurate medication list at hospital admission in particular is vital when evaluating patients’ current pharmacotherapy and in determining further treatment options. A cost-effectiveness evaluation indicated that discharge counseling by pharmacists was cost saving in 48% of scenarios, but all scenarios were cost-effective at a low willingness-to-pay value. High-risk elderly patients appeared to benefit most from this service. It has been shown that pharmacists’ involvement at admission and discharge has resulted in reduced medication errors and ADEs, as well as a substantial decrease in the rate of all-cause ED visits and hospital readmissions. Furthermore, pharmacist-led reconciliation has been shown to have the highest expected cost benefits when compared with other reconciliation processes (Dalton et.al., 2017; Sawyer et.al., 2017)
CONCLUSION
PE evaluation has become an important area of interest to find the optimal therapy at the lowest price as healthcare resources are not easily accessible and affordable to many patients. Numerous drug alternatives and empowered consumers also fuel the need for economic evaluations of pharmaceutical products. In developing countries, the PE can help the poor and middle class to obtain well health care services because many households are below poverty line, unaffordable for private health care. Costs of the medicines are constantly growing. In countries with scarce resources and an ever-growing population with diverse health care needs, an innovative method called, pharmacoeconomic evaluation plays an essential role in determining the delivery of reasonable and cost-effective health services. Applied PE has been lacking as the most vital practice of pharmacy. Understanding the principles, methods, and application of PE, enables pharmacists to make healthier, more informed judgments concerning the use of pharmaceutical goods and services. Specifically, decisions that ultimately represent the best welfares of the patient, the healthcare system, and society. PE applied to any therapeutic area like hospital pharmacy, using a variety of application plans.
REFERENCES
1. American Pharmacists Association (APhA), (2008); National Association of Chain Drug Stores Foundation. Medication Therapy Management Services. J Am Pharm Assoc. ; 48(3); 341-53
2. Ambrosioni E. (2001); Pharmacoeconomic challenges in disease management of hypertension. J Hypertens Suppl. ;19(3); S33-40
3. Annual Report Of The Boards Of Trustees, (2012); Available From: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/ReportsTrustFunds/downloads/tr2012.pdf
4. Ayele AA, Mekuria AB, Tegegn HG, Gebresillassie BM, Mekonnen AB, Erku DA, (2018); Management of minor ailments in a community pharmacy setting: Findings from simulated visits and qualitative study in Gondar town, Ethiopia. PLoS One.;13(1); e0190583
5. Auta A, Maz J, Strickland-Hodge B. (2015); Perceived facilitators to change in hospital pharmacy practice in England. Int J Clin Pharm. ; 37(6); 1068–1075.
6. Beijer HJ, de Blaey CJ. (2002); Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. ; 24(2); 46–54.
7. Bunting BA, Smith BH, Sutherland SE (2008); The Asheville Project: clinical and economic outcomes of a community-based long-term medication therapy management program for hypertension and dyslipidemia. J Am Pharm Assoc (2003) ; 48(1); 23–31.
8. Bekker CL, Gardarsdottir H, Egberts ACG, Bouvy ML, van den Bemt BJF. (2018); Pharmacists' Activities to Reduce Medication Waste: An International Survey. Pharmacy (Basel). ; 6(3); 94.
9. Bettington E, Spinks J, Kelly F, Wheeler AJ (2018); Returning unwanted medicines to pharmacies: prescribing to reduce waste. Aust Prescr ; 41(3) ; 78-81.
10. Babigumira, J.B., Stergachis, A., Choi, H.L. et al. (2014); A Framework for Assessing the Economic Value of Pharmacovigilance in Low- and Middle-Income Countries ; Drug Saf ;37(3); 127-34
11. Chabot I, LeLorier J, Blackstein ME. (2008); The challenge of conducting pharmacoeconomic evaluations in oncology using crossover trials: the example of sunitinib for gastrointestinal stromal tumour. Eur J Cancer. ;44(7); 972-977
12. Catić T, Skrbo S. (2013); Pharmacoeconomic education for pharmacy students in bosnia and herzegovina. Mater Sociomed. ; 25(4); 282-5.
13. Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. (2018); Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. ;8(1); e016982.
14. Dalton K, Byrne S. (2017); Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. ; 6; 37-46
15. Dubois DJ. (2010); Grand challenges in pharmacoeconomics and health outcomes. Front Pharmacol. ; 1:7.
16. Drummond M. (2006); Pharmacoeconomics: friend or foe? Annals of the Rheumatic Diseases ; 65; iii44-iii47.
17. Gyllensten H, Hakkarainen KM, Hägg S, et al. (2014); Economic impact of adverse drug events--a retrospective population-based cohort study of 4970 adults. PLoS One. ;9(3):e92061.
18. Gattani SG, Patil AB, Kushare SS. (2009); Pharmacoeconomics: A Review. Asian Journal of Pharmaceutical and Clinical Research; 2(3); July-September
19. Hall D, Buchanan J, Helms B, et al. (2011); Health care expenditures and therapeutic outcomes of a pharmacist-managed anticoagulation service versus usual medical care. Pharmacotherapy. ;31(7); 686–694.
20. Henry D, Taylor C. (2014); Pharmacoeconomics of Cancer Therapies: Considerations With the Introduction of Biosimilars. Seminars in Oncology; 41(2); Suppl 3, S13-S20
21. IMS Institute for Healthcare Informatics (2015). Global Medicines use in 2020: Outlook and Implications. Available From: https://s3.amazonaws.com/assets.fiercemarkets.net/public/005-LifeSciences/imsglobalreport.pdf.
22. James TH, Helms ML, Braund R. (2009); Analysis of medications returned to community pharmacies. Ann Pharmacother. ; 43(10); 1631-1635
23. Jo C. (2014); Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol; 20(4):327-337
24. Khdour MR, Kidney JC, Smyth BM, McElnay JC. (2009); Clinical pharmacyled disease and medicine management programme for patients with COPD. Br J Clin Pharmacol. ; 68(4); 588–598.
25. Lee JK, Grace KA, Taylor AJ. (2006); Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. ;296(21); 2563–2571.
26. Latif A. (2018); Community pharmacy Medicines Use Review: current challenges. Integr Pharm Res Pract. ; 7; 83-92
27. Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. (2008); Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. ; 168(17); 1890–1896.
28. Morello CM, Zadvorny EB, Cording MA, Suemoto RT, Skog J, Harari A. (2006); Development and clinical outcomes of pharmacist-managed diabetes care clinics. Am J Health Syst Pharm. ;63(14); 1325–1331.
29. Milne RJ. (1994); Evaluation of the pharmacoeconomic literature. Pharmacoeconomics. ;6(4); 337-45.
30. McGhan WF, Rowland CR, Bootman JL. (1978); Cost-benefit and cost-effectiveness: Methodologies for evaluating innovative pharmaceutical services. Am J Hosp Pharm. ; 35(2); 133–40.
31. NHS (2005). United-Kingdom Department of Health: Choosing health through pharmacy–a programme for pharmaceutical public health 2005–2015
32. Parkis R. Pharmacoeconomics - the importance for pharmacists. The Pharmaceutical Journal FEB 2006.
33. Rotta I, Salgado TM, Silva ML, Correr CJ, Fernandez-Llimos F. (2015); Effectiveness of clinical pharmacy services: an overview of systematic reviews (2000-2010) Int J Clin Pharm. ; 37(5); 687–697.
34. Sawyer RT, Odom JM, Jennings J, Orr J, Cass AL. (2016); Discharge medication reconciliation by pharmacists to improve transitions following hospitalization (DEPTH); GHS Proc ; 1(1); 32-37
35. Systems for Improved Access to Pharmaceuticals and Services (SIAPS) and USAID. Applying Principles of Pharmacoeconomics to Improve Medical Product Selection and Use in Low- and Middle-income Countries: Trainer’s Guide. http://siapsprogram.org/ August 2017.
36. Surji KM. (2015); Fundamental Understanding of Pharmacoeconomics as an Innovative Concept within the Modern Clinical Pharmacy in Today’s Healthcare System American Journal of Pharmacy and Health Research ; 3(5) ISSN: 2321–3647
37. TRASK L. Chapter 1. Pharmacoeconomics: Principles, Methods, and Applications. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 8e New York, NY: McGraw-Hill; 2011. http://accesspharmacy.mhmedical.com/content.aspx?bookid=462§ionid=41100767. Accessed January 25, 2019.
38. Telser H, Fischer B, Leukert K, Vaterlaus S. Healthcare expenditure and illness-related costs. In InterPharmaPh Polynomics. Web Interpharma (Association of research-based pharmaceutical companies in Switzerland, Basel) September 2011. Available From: https://www.interpharma.ch/sites/default/files/healthcare_expenditure_illness_related_costs_2011.pdf
39. Tinetti ME, Fried TR, Boyd CM. (2012); Designing health care for the most common chronic condition: multimorbidity. JAMA. ; 307(23); 2493–2494.
40. Usherwood T. (2017); Encouraging adherence to long-term medication. Aust Prescr. ; 40(4); 147-150.
41. Vogenberg FR, Santilli J. (2018); Healthcare Trends for 2018. Am Health Drug Benefits. ; 11(1); 48-54.
42. Waning B, Montagne M. Chapter 9. Principles of Pharmacoeconomics. In: Brenda Waning, Michael Montagne. Pharmacoepidemiology: Principles and Practice, published by McGraw-Hill, 2001
43. National Library of Canada (1997). Canadian Coordinating Office for Health Technology Assessment. Guidelines For Economic Evaluation Of Pharmaceuticals 2nd Edition: Canada Available From: https://www.cadth.ca/media/pdf/peg_e.pdf
44. Web Centers for Medicare & Medicaid Service (CMS). National Health Expenditure Data. Available From: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html
45. Web Pharmadost (2017); Pharmacoeconomics: Evaluation methods Available From: https://pharmadost.info/pharmacoeconomics-evaluation-methods/
46. Web Centers for Medicare and Medicaid Services (CMS). NHE Projections 2010–2020. Washington, DC: US Department of Health and Human Services; 2011
47. Web Henry J Kaiser Family Foundation (2012); Health care costs: a primer. Available from: http:www.kff.org/insurance/upload/7670-7603.pdf.
48. Wheeler AJ, Spinks J, Bettington E, Kelly F. (2017); Evaluation of the National Return of unwanted medicines (RUM) program in Australia: a study protocol. J Pharm Policy Pract; 10; 38.
49. Yach D, Hawkes C, Gould CL, Hofman KJ. (2004); The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA. ; 291(21); 2616–2622.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









