ABOUT AUTHORS:
Patil Reshma R.*, BhutkarM.A., Gaikwad Pranita T.
Rajarambapu College of Pharmacy, Kasegaon,
Maharashtra 415405 India
*Patil.reshma1990@gmail.com
ABSTRACT:
Peripheral vascular disease (PVD) is a slow and progressive circulation disorder. It may involve disease in any of the blood vessels outside of the heart and diseases of the lymph vessels - the arteries, veins, or lymphatic vessels. Organs supplied by these vessels such as the brain, heart, and legs, may not receive adequate blood flow for ordinary function. However, the legs and feet are most commonly affected, thus the name peripheral vascular disease. In the US, about 8 million people have peripheral artery disease. The prevalence of peripheral arterial disease is dependent upon patient age and the presence of traditional risk factors for cardiovascular disease. Many patients are undiagnosed and are at substantial risk for coronary and cerebrovascular events. As with any atherosclerotic condition, several risk factors play an important role in the morbidity and mortality of peripheral vascular disease. Many of these risk factors can be modified with the help of various nonpharmacologic and pharmacologic interventions.
REFERENCE ID: PHARMATUTOR-ART-1884
What is peripheral vascular disease (PVD)?
Peripheral vascular disease (PVD) is a slow and progressive circulation disorder. It may involve disease in any of the blood vessels outside of the heart and diseases of the lymph vessels - the arteries, veins, or lymphatic vessels. Organs supplied by these vessels such as the brain, heart, and legs, may not receive adequate blood flow for ordinary function. However, the legs and feet are most commonly affected, thus the name peripheral vascular disease.
Peripheral vascular disease (PVD), commonly referred to as peripheral artery disease (PAD) or peripheral artery occlusive disease (PAOD).
Causes
Risk factors contributing to PAD are the same as those for atherosclerosis:[1][4]
- Smoking - tobacco use in any form is the single most important modifiable cause of PVD internationally. Smokers have up to a tenfold increase in relative risk for PVD in a dose-related effect.[4] Exposure to second-hand smoke from environmental exposure has also been shown to promote changes in blood vessel lining (endothelium) which is a precursor to atherosclerosis.Smokers are 2 to 3 times more likely to cause lower extremity peripheral arterial disease than coronary artery disease.[5] More than 80%-90% of patients with lower extremity peripheral arterial disease are current or former smokers.[6] The risk of PAD increases with the number of cigarettes smoked per day and the number of years smoked.[7][8]
- Diabetes mellitus - causes between two and four times increased risk of PVD by causing endothelial and smooth muscle cell dysfunction in peripheral arteries.[9][10][11] The risk of developing lower extremity peripheral arterial disease is proportional to the severity and duration of diabetes.[12] Diabetics account for up to 70% of nontraumatic amputations performed, and a known diabetic who smokes runs an approximately 30% risk of amputation within 5 years.
- Dyslipidemia (high low density lipoprotein [LDL] cholesterol, low high density lipoprotein [HDL] cholesterol) - elevation of total cholesterol, LDL cholesterol, and triglyceride levels each have been correlated with accelerated PAD. Correction of dyslipidemia by diet and/or medication is associated with a major improvement in rates of heart attack and stroke.[13] This benefit is gained even though current evidence does not demonstrate a major reversal of peripheral and/or coronary atherosclerosis.
- Hypertension - elevated blood pressure is correlated with an increase in the risk of developing PAD, as well as in associated coronary and cerebrovascular events (heart attack and stroke).Hypertension increased the risk of intermittent claudication 2.5- to 4-fold in men and women,respectively[14]
- Risk of PAD also increases in individuals who are over the age of 50, male, obese, heart attack, or stroke.[15][16] or with a family history of vascular disease[17][18]
Epidemiology:
The prevalence of peripheral vascular disease in the general population is 12–14%, affecting up to 20% of those over 70;[20] 70%–80% of affected individuals are asymptomatic; only a minority ever require revascularisation or amputation. Peripheral vascular disease affects 1 in 3 diabetics over the age of 50.
In the USA peripheral arterial disease affects 12–20 percent of Americans age 65 and older. Approximately 10 million Americans have PVD. Despite its prevalence and cardiovascular risk implications, only 25 percent of PAD patients are undergoing treatment.
The incidence of symptomatic PVD increases with age, from about 0.3% per year for men aged 40–55 years to about 1% per year for men aged over 75 years. The prevalence of PVD varies considerably depending on how PAD is defined, and the age of the population being studied. Diagnosis is critical, as people with PAD have a four to five time’s higher risk of heart attack or stroke.
The risk of death is approximately the same in men and women and is elevated even in asymptomatic patients. Annual mortality is 25% in patients with critical leg ischemia who have the lowest ABI values.[10]
The Diabetes Control and Complications Trial and U.K. Prospective Diabetes Study trials in people with type 1 and type 2 diabetes, respectively, demonstrated that glycemic control is more strongly associated with microvascular disease than macrovascular disease. It may be that pathologic changes occurring in small vessels are more sensitive to chronically elevated glucose levels than is atherosclerosis occurring in larger arteries.[21]
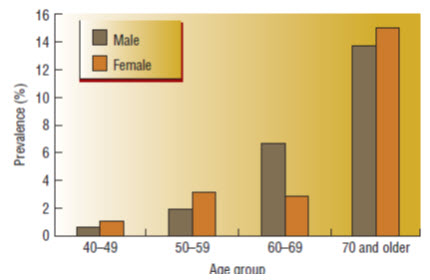
Fig.1-Prevalence of peripheral arterial disease by age and gender
CONDITIONS ASSOCIATED WITH PVD :
- Conditions associated with PVD that affect the veins include deep vein thrombosis (DVT), varicose veins, and chronic venous insufficiency.
- Lymphedema is an example of PVD that affects the lymphatic vessels.
- When PVD occurs in the arteries outside the heart, it may be referred to as peripheral arterial disease (PAD). Conditions associated with PAD may be occlusive (occurs because the artery becomes blocked in some manner) or functional (the artery either constricts due to a spasm or expands). Examples of occlusive PAD include peripheral arterial occlusion and Buerger's disease (thromboangiitis obliterans). Examples of functional PAD include Raynaud's disease and phenomenon and acrocyanosis. However, the terms "peripheral vascular disease" and "peripheral arterial disease" are often used interchangeably. In the US, about 8 million people have peripheral artery disease. It is frequently found in people with coronary artery disease, because atherosclerosis, which causes coronary artery disease, is a widespread disease of the arteries.[28]
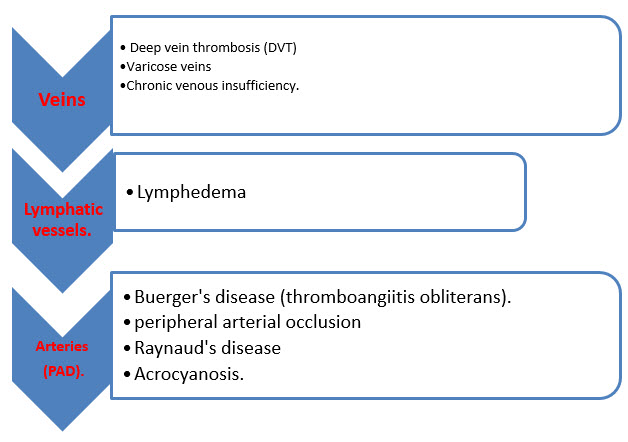
Fig.2 - Conditions associated with Peripheral vascular diseases
Fig 3- Peripheral Arterial diseases
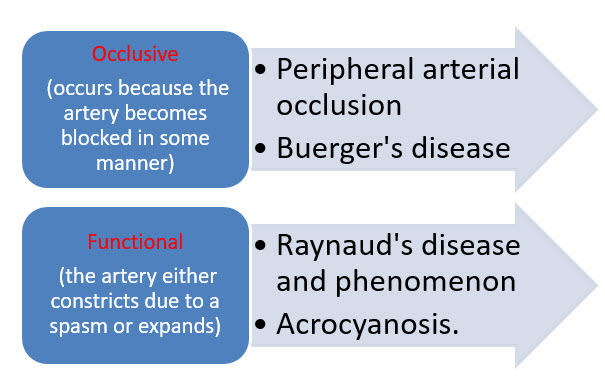
Fig.4-Conditions associated with Peripheral arterial diseases
The term "peripheral vascular disease" encompasses several different conditions. Some of these conditions include, but are not limited to, the following:
- Atherosclerosis - the build-up of plaque inside the artery wall. Plaque is made up of deposits of fatty substances, cholesterol, cellular waste products, calcium, and fibrin. The artery wall then becomes thickened and loses its elasticity. Symptoms may develop gradually, and may be few, as the plaque builds up in the artery. However, when a major artery is blocked, a heart attack, stroke, aneurysm, or blood clot may occur, depending on where the blockage occurs.
- Buerger's disease (thromboangiitis obliterans) - a chronic inflammatory disease in the peripheral arteries of the extremities leading to the development of clots in the small- and medium-sized arteries of the arms or legs and eventual blockage of the arteries. Buerger's disease most commonly occurs in men between the ages of 20 and 40 who smoke cigarettes. Symptoms include pain in the legs or feet, clammy cool skin, and a diminished sense of heat and cold.
- Chronic venous insufficiency - a prolonged condition in which one or more veins do not adequately return blood from the lower extremities back to the heart due to damaged venous valves. Symptoms include discoloration of the skin and ankles, swelling of the legs, and feelings of dull aching pain, heaviness, or cramping in the extremities.
- Deep vein thrombosis (DVT) - a clot that occurs in a deep vein, and has the potential to dislodge, travel to the lungs, occlude a lung artery (pulmonary embolism), and cause a potentially life-threatening event. It is found most commonly in those who have undergone extended periods of inactivity, such as from sitting while traveling or prolonged bed rest after surgery. Symptoms may be absent or subtle, but include swelling and tenderness in the affected extremity, pain at rest and with compression, and raised vein pattern.
- Raynaud's phenomenon - a condition in which the smallest arteries that bring blood to the fingers or toes constrict (go into spasm) when exposed to cold or as the result of emotional upset. Raynaud's most commonly occurs in women between the ages of 18 and 30. Symptoms include coldness, pain, and pallor (paleness) of the fingertips or toes.
- Thrombophlebitis - a blood clot in an inflamed vein, most commonly in the legs, but it can also occur in the arms. The clot can either be close to the skin (superficial thrombophlebitis) or deep within a muscle (deep vein thrombosis). It may result from pooling of blood, venous wall injury, and altered blood coagulation. Symptoms in the affected extremity include swelling, pain, tenderness, redness, and warmth.
- Varicose veins - dilated, twisted veins caused by incompetent valves (valves that allow backward flow of blood) allowing blood to pool. It is most commonly found in the legs or lower trunk. Symptoms include bruising and sensations of burning or aching. Pregnancy, obesity, and extended periods of standing intensify the symptoms.
SYMPTOMS[23]:
Approximately half the people diagnosed with peripheral vascular disease are symptom free. For those experiencing symptoms, the most common first symptom is intermittent claudication in the calf (leg discomfort described as painful cramping that occurs with exercise and is relieved by rest). During rest, the muscles need less blood flow, so the pain disappears. It may occur in one or both legs depending on the location of the clogged or narrowed artery.
Other symptoms of peripheral vascular disease may include:
- changes in the skin, including decreased skin temperature, or thin, brittle shiny skin on the legs and feet
- diminished pulses in the legs and the feet
- gangrene (dead tissue due to lack of blood flow)
- hair loss on the legs
- impotence
- non-healing wounds over pressure points, such as heels or ankles
- numbness, weakness, or heaviness in muscles
- pain (described as burning or aching) at rest, commonly in the toes and at night while lying flat
- pallor (paleness) when the legs are elevated
- reddish-blue discoloration of the extremities
- restricted mobility
- severe pain
- thickened, opaque toenails
The symptoms of peripheral vascular disease may resemble other conditions. Consult your physician for a diagnosis.
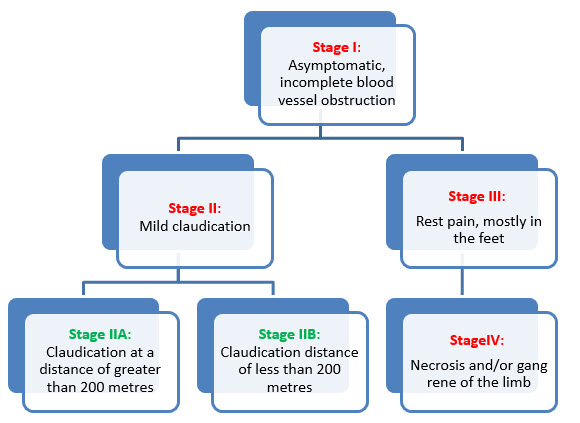
Fig. 5- Stages of peripheral vascular diseases introduced by René Fontaine in 1954
DIAGNOSIS:
In addition to a complete medical history and physical examination, diagnostic procedures for peripheral vascular disease may include any, or a combination, of the following:
- Angiogram - an x-ray of the arteries and veins to detect blockage or narrowing of the vessels. This procedure involves inserting a thin, flexible tube into an artery in the leg and injecting a contrast dye. The contrast dye makes the arteries and veins visible on the x-ray[1,20].
- Ankle-brachial index (abi) - a comparison of the blood pressure in the ankle with the blood pressure in the arm using a regular blood pressure cuff and a doppler ultrasound device. To determine the abi, the systolic blood pressure (the top number of the blood pressure measurement) of the ankle is divided by the systolic blood pressure of the arm. ABPI/ABI). When the blood pressure readings in the ankles is lower than that in the arms, blockages in the arteries which provide blood from the heart to the ankle are suspected. Normal ABI range of 1.00 to 1.40.The patient is diagnosed with PAD when the ABI is ≤ 0.90 . ABI values of 0.91 to 0.99 are considered ‘‘borderline’’ and values >1.40 indicate noncompressible arteries. PAD is graded as mild to moderate if the ABI is between 0.41 and 0.90, and an ABI less than 0.40 is suggestive of severe PAD[8].
- Blood lipid profile - a blood test to measure the levels of each type of fat in your blood: total cholesterol, ldl cholesterol, hdl cholesterol, triglycerides, and others.
- Doppler ultrasound flow studies - uses high-frequency sound waves and a computer to create images of blood vessels, tissues, and organs. Doppler technique is used to measure and assess the flow of blood. Faintness or absence of sound may indicate an obstruction in the blood flow.
- Magnetic resonance angiography (mra) - a noninvasive diagnostic procedure that uses a combination of a large magnet, radiofrequencies, and a computer to produce detailed images of organs and structures within the body. An mra is often used to examine the heart and other soft tissues and to assess blood flow. The advantages of MRA include its safety and ability to provide high-resolution three-dimensional (3D) imaging of the entire abdomen, pelvis and lower extremities in one setting.[29]
- Treadmill exercise test - a test that is given while a patient walks on a treadmill to monitor the heart during exercise.[22]
- Photoplethysmography (ppg) - an examination comparable to the ankle brachial index except that it uses a very tiny blood pressure cuff around the toe and a ppg sensor (infrared light to evaluate blood flow near the surface of the skin) to record waveforms and blood pressure measurements. These measurements are then compared to the systolic blood pressure in the arm.
- Pulse volume recording (pvr) waveform analysis - a technique used to calculate blood volume changes in the legs using a recording device that displays the results as a waveform.
- Reactive hyperemia test - a test similar to an abi or a treadmill test but used for people who are unable to walk on a treadmill. While a person is lying on his or her back, comparative blood pressure measurements are taken on the thighs and ankles to determine any decrease between the two sites.
- Segmental blood pressure measurements - a means of comparing blood pressure measurements using a doppler device in the upper thigh, above and below the knee, at the ankle, and on the arm to determine any constriction in blood flow.
TREATEMENT:
GENERAL APPROACH TO TREATMENT
As with any atherosclerotic condition, several risk factors play an important role in the morbidity and mortality of PVD. Many of these risk factors can be modified with the help of various nonpharmacologicand pharmacologic interventions.
NONPHARMACOLOGIC THERAPY
Smoking Cessation
Cigarette smoking not only increases the risk of developing PAD and other cardiovascular disorders, but the duration and quantity smoked can negatively impact disease progression (i.e., increase the risk of amputation) and increase mortality.[22] Numerous studies have proved the effectiveness of individual or group behavior modification therapy with or without the addition of certain antidepressants (e.g., bupropion) or nicotine replacement therapies (e.g., gum or patches). Reassessment of smoking status and progress encouragement at each visit can help reemphasize to the patient the vital importance of this lifestyle change[4.5,7,8].
Exercise
Walking exercise programs for patients with PAD have been proven to result in increased walking duration and distance, increased painfree walking, and delayed onset of claudication by 179%.22 Walking, or any aerobic exercise program conducted under the supervision of a healthcare provider, can positively impact several of the pathophysiologic abnormalities present in patients with PVD.
Benefits of exercise programs include improving diabetes and lipid management, reducing weight, improving blood viscosity and flow, and reducing blood pressure. The American College of Cardiology/ American Heart Association Guidelines for the Management of PVD recommend supervised exercise training for patients with intermittent claudication, for a minimum of 30–45 minutes, performed at least three times per week. A prospective, observational study has concluded that PAD patients with higher physical activity (as measured with a vertical accelerometer) have reduced mortality and cardiovascular events compared to those with low physical activity, regardless of confounders.49 Exercise treadmill walking testing should be repeated at regular intervals (i.e., quarterly to biannually) to assess improvement or decline in walking duration and distance as well as time to pain onset while performing this activity.
Revascularization Therapy
Various surgical procedures are available for patients with severe, debilitating claudication who have attempted, and failed, other means of nonpharmacologic and pharmacologic therapy. The TransAtlantic Inter-Society Consensus (TASC) document on PAD provides clear recommendations for invasive therapy. [25]
First, the patient must have a lack of adequate response to exercise therapy and risk factor modification. Second, the patient must have severe disability from intermittent claudication resulting in impairment of daily activities. Third, a thorough evaluation of the risks versus benefits of an invasive intervention must be preformed, including probability of success, anticipated future course of the disease if intervention is not performed, and evaluation of concomitant disease states. [25]
Although the TASC is clear that revascularization is mostly reserved for those who do not respond to conservative therapy consisting of risk factor modification, exercise, and pharmacologic therapy, revascularization does have a role in patients with favorable anatomy, whose lifestyle and/or job performance are compromised and who have a favorable risk-to-benefit ratio based on procedural risk.12 The decision to attempt percutaneous revascularization often is made with the guidance of diagnostic angiography. Angiography can help to identify the location and size of lesions and provide valuable information regarding the likelihood of success with surgical revascularization.[25]
Percutaneous transluminal angioplasty (PTA) is an example of an invasive treatment of PAD. A randomized controlled clinical trial by Whyman et al. determined that in a 2-year postintervention, PTA outcomes on maximum walking distance and ABI were not significantly different than outcomes in patients who had received only daily low-dose ASA (P >0.05). Nevertheless, patients who had received PTA had significantly fewer occluded arteries (P = 0.003), but the true clinical significance of this finding was not realized in the time allotted for the study. PTA typically is reserved for patients whose lifestyle and/ or job performance are compromised secondary to claudication despite adequate pharmacologic interventions and exercise.12,38 Stent placement in PAD patients has been an area of study and controversy. A meta-analysis examining the use of stent placement versus PTA for treatment of aortoiliac occlusive disease determined that although stent placement and PTA yielded similar complication
and mortality rates, posttreatment ABI was more improved with stents (0.87 with PTA and 0.76 with stents, P <0.03) and the risk of long-term failure was 39% less with stent placement.51 However, other studies have not demonstrated improvement in patency rates in peripheral arteries versus PTA alone.[20] The TASC document provides specific recommendations for PTA, with or without stenting, depending on the how diffuse the disease process is, the number and size of the lesions, and the location of the lesions.[25]
PHARMACOLOGIC THERAPY
Management of Hypertension
Hypertension is a major risk factor for PAD and can lead to acute myocardial infarction, stroke, heart failure, and death.[26] Current guidelines recommend that the treatment goal for blood pressure in patients with PAD mirror those in patients with documented CVD: 130/85 mm Hg. Although, the Heart Outcomes Prevention Evaluation (HOPE) study demonstrated that angiotensin-converting enzyme inhibitors reduced not only blood pressure but other cardiovascular events (e.g., acute myocardial infarction, stroke, and death) in high-risk patients, including those with PAD, no specific class of antihypertensives is recommended over another for treatment of hypertension in patients with PAD. Therefore, selection of drug therapy for hypertension should be made on the basis of comorbid disease states, drug costs and availability, drug allergies, or other possible limiting factors. For example, patients with documented CAD may receive a dual benefit by the selection of a β- blocker, whereas patients with concomitant Raynaud phenomenon may benefit from calcium channel blockers.[26]
Management of Hyperlipidemia
Although a reduction in lipid levels can reduce the progression of PVD and the severity of claudication, the current recommendations for management of hyperlipidemia in patients with PVD are based on only a few small studies and sub hoc analyses from larger trials.The Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) considersPVD to be in the category of highest risk, or a coronary heart disease risk equivalent. Therefore, the Expert Panel recommends that LDL levels be maintained at <100 mg/dL and non–high-density lipoprotein (HDL) levels (total cholesterol – HDL cholesterol) maintained at <130 mg/dL. Results of clinical trials conducted since issuance of the Expert Panel recommendation, specifically the Heart Protection Study (HPS)[27] and the Pravastatin or Atorvastatin Evaluation and Infection—Thrombolysis in Myocardial Infarction (PROVE IT) trial, have led many clinical experts to now recommend an LDL goal of <70 mg/dL for additional retardation of atherosclerotic plaque formation in persons considered to be at very high risk, including patients with PAD.[20] Regardless of the goal LDL level chosen, initiation of patient therapeutic lifestyle changes (e.g., reduction in consumption of saturated fat, weight reduction, and increased physical activity) is vital to achieving these recommendations.[27] Unfortunately, in many cases, therapeutic lifestyle changes alone will not achieve the desired goals.Several options are available for initiating drug therapy to lower LDL levels in patients with PAD. Statins, bile acid sequestrants, and nicotinic acid all are effective treatment options. However, statins are the preferred starting agent in this patient population.[14,27] As proven in the HPS, simvastatin demonstrated potent action in reducing LDL and also provided a significant reduction in cardiovascular events overall (e.g., acute myocardial infarction, stroke, and death). If an increase in HDL levels also is necessary, niacin should be considered alone or in combination with a statin without the fear of worsening glucose metabolism, as previously believed.[8,22]
Management of Diabetes Mellitus
A meta-analysis of more than 95,000 diabetic patients provided additional support for the accepted premise that glycemic control serves as a risk factor for CVD. [11] The analysis demonstrated an increasing risk of death from cardiovascular events as blood glucose concentrations increased, with the same relationship observed even at levels below the threshold of clinically defined diabetes mellitus. This relationship is just one illustration of the criticality of good glycemic control. Due to the high prevalence of PVD among diabetic patients, the American Diabetes Association recommends ABI screening for PVD in all diabetics older than 50 years.[12] Because of the presence of peripheral neuropathy, patients with diabetes may be less likely to experience or report symptoms of PVD, and the first sign may be as drastic as the appearance of a gangrenous foot ulcer. Therefore, although a lack of randomized controlled studies illustrates that the degree of glycemic control is predictive of the extent of PAD present, it is widely recommended that all patients with concomitant diabetes and PAD maintain good glycemic control, as evidenced by a hemoglobin A1c level <7%.This recommendation is supported by a prospective cohort study of 1,894 diabetic patients. The study demonstrated that patients with poor glucose control (hemoglobin A1c >7.5%) were five times more likely to develop intermittent claudication and to be hospitalized for PAD compared to patients with hemoglobin A1c <6%. Despite this finding, a study by Rehring et al. of 365 patients with known PAD and concomitant diabetes showed that only 45.8% of these patients had hemoglobin A1c <7%. [13]
ANTIPLATELET DRUG THERAPY
Aspirin
By far, the most compelling evidence for the use of any pharmacologic agent in patients with PAD can be found for aspirin (acetylsalicylic acid [ASA]). The Antithrombotic Trialists’ Collaboration (ATC) conducted a meta-analysis of 195 randomized trials, composed of more than 135,000 patients at high risk for occlusive arterial disease. The ATC concluded that low-dose ASA (75–160 mg) and mediumdose ASA (160–325 mg/day) led to a significant reduction in serious vascular events (12%) in “high-risk” patients, such as those with PAD.[23] The ATC also noted in this analysis that the risk of major extracranial bleed was similar between low-dose and medium-dose regimens.[25]
ASA + Dipyridamole
The ATC also examined the use of dipyridamole extended-release (Aggrenox) in combination with ASA in “high-risk” patients, such as those with PAD. The meta-analysis of trials (which included more than 10,000 patients) concluded that the addition of dipyridamole to aspirin led to a further reduction in serious vascular events over ASA alone (6%); however, this reduction did not reach statistical significance (P = 0.32). Also to be taken into consideration is that most of the reduction in nonfatal stroke in this analysis came from one trial, and these data have not been replicated in other studies.
Clopidogrel (Plavix)
The ATC meta-analysis also reviewed the effectiveness of clopidogrel 75 mg/day in “high-risk” patients, including those with PAD. The ATC concluded that although clopidogrel was able to reduce serious vascular events by 10%, this was significantly less than the reduction seen with ASA (12%, P = 0.03) described previously. Included in the meta-analysis was the report from the Clopidogrel versus ASA in Patients at Risk of Ischemic Events (CAPRIE) trial, which concluded that clopidogrel 75 mg/day was more effective than ASA 325 mg/day in preventing vascular events in “high-risk” patients. In comparison to ASA therapy, the clopidogrel regimen resulted in an overall reduction in ischemic stroke, myocardial infarction, or vascular death from 5.83% to 5.32% (P = 0.043). This difference was even more pronounced in the subgroup analysis of PAD patients, in which clopidogrel therapy led to a significant reduction of 4.86% versus 3.71% in the ASA group (P = 0.0028). It must be noted that clopidogrel is significantly more expensive than ASA therapy, not only in terms of drug costs but also in cost of physician visits because clopidogrel is a prescription-only medication. For all these reasons, the current recommendations list clopidogrel as a first-line agent, but only in cases where ASA therapy is either not tolerated or contraindicated.[28.23]
Ticlopidine (Ticlid)
Although ticlopidine has the same mechanism of action as clopidogrel and possesses a similar molecular structure, the results of clinical trials investigating the two agents are strikingly different.[22] The Swedish Ticlopidine Multicenter Study (STIMS) had determined that ticlopidine therapy (500 mg/day) reduced total mortality in patients with intermittent claudication in comparison to placebo (P = 0.015). However, the once promising results seen with ticlopidine therapy now have been overshadowed by the severe hematologic side effects unique to this agent. Ticlopidine has a black box warning from the Food and Drug Administration (FDA) warning providers that use of this agent can cause neutropenia/agranulocytosis, thrombotic thrombocytopenic purpura, and aplastic anemia.
Treatment with other drugs or vitamins are unsupported by clinical evidence, "but trials evaluating the effect of folate and vitamin B-12 on hyperhomocysteinaemia, a putative vascular risk factor, are near completion".[23]
|
Drug |
Dose |
Mechanism of action |
Side effects |
Contrindications |
|
Aspirin |
81–325 mg |
Irreversibly inhibits prostaglandin cyclooxygenase in platelets, prevents formation of thromboxane A2 |
Gastrointestinal upset and/or bleeding
|
Active bleeding; hemophilia; thrombocytopenia |
|
Dipyridamole extended-release (Aggrenox) |
400 mg (+ aspirin 50 mg) |
May act by inhibiting platelet aggregation (complete mechanism of action unknown) |
Angina, dyspnea, hypotension, headache, dizziness |
Active bleeding; coronary artery disease (“coronary steal syndrome”) |
|
Cilostazol (Pletal)a |
100 mg bid |
Phosphodiesterase inhibitor, suppresses platelet aggregation; direct artery vasodilator |
Fever from infection, tachycardia |
All congestive heart failure patients (decreased survival) |
|
Clopidogrel (Plavix)
|
75 mg |
Inhibits binding of ADP analogues to its platelet receptor, causing irreversible inhibition of platelets |
Chest pain, purpura generalized pain, rash |
Active pathologic bleeding (e.g., peptic ulcer, intracranial hemorrhage) |
|
Pentoxifylline (Trental) |
1.2 g |
Alters red blood cell flexibility; decreases platelet adhesion; reduces blood viscosity; decreases fibrinogen concentration |
Dyspnea, nausea, vomiting, headache, dizziness |
Recent retinal or cerebral hemorrhage; active bleeding |
|
Ticlopidine (Ticlid) |
500 mg |
Inhibits binding of ADP analogues to its platelet receptor, causing irreversible inhibition of platelets |
Leukopenia; rash; thrombocytopenia;neutropenia, agranulocytosis; aplastic anemia |
Active bleeding, hemophilia; thrombocytopenia |
Table 1- some drugs used in the treatment of PVD
REFERENCES:
1.Peripheral Arterial Disease at Merck Manual of Diagnosis and TherapyProfessional Edition Retrieved on August 9, 2010
2.Fontaine R, Kim M, Kieny R (1954). "Die chirugische Behandlung der peripheren Durchblutungsstörungen. (Surgical treatment of peripheral circulation disorders)".Helvetica Chirurgica Acta (in German) 21 (5/6): 499–533
3.Norgren L, Hiatt WR, Dormandy JA (2007). "Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II)". Eur J Vasc Endovasc Surg. 33(Suppl 1): S1 75. Norgren L, Hiatt WR, Dormandy JA, TASC II Working Group et al. (2007). "Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II)". J Vasc Surg. 45 (Suppl S): S5–67
4.Norgren L, Hiatt WR, Dormandy JA (2007). "Inter-Society Consensus for the Management of Peripheral Arterial Disease". Int Angiol. 26 (2): 81–157..
5.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012 Oct 24;308(16):1660-7. doi: 10.1001/jama.2012.13415
6.Price J, Mowbray P, Lee A, Rumley A, Lowe G, Fowkes F: Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease; Edinburgh Artery Study Edinburgh Artery Study. European heart journal 1999, 20(5):344-353.
7.Smith GD, Shipley M, Rose G: Intermittent claudication, heart disease risk factors, and mortality. The Whitehall Study. Circulation 1990, 82(6):1925-1931.
8.Cole C, Hill G, Farzad E, Bouchard A, Moher D, Rody K, Shea B: Cigarette smoking and peripheral arterial occlusive disease. Surgery 1993, 114(4):753.
9.Hirsch AT, Haskal ZJ, Hertzer NR et al. (2006). . J. Am. Coll. Cardiol. 47 (6): 1239312.
10.Kannel WB, McGee D: Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes care 1979, 2(2):120-126.
11.Creager MA, Lüscher TF, Cosentino F, Beckman JA: Diabetes and vascular disease pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003, 108(12):1527-1532.
12.Lüscher TF, Creager MA, Beckman JA, Cosentino F: Diabetes and vascular disease pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 2003, 108(13):1655-1661.
13.Beks P, Mackaay A, De Neeling J, De Vries H, Bouter L, Heine R: Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn study. Diabetologia 1995, 38(1):86-96.
14.Unit ES: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005, 366:1267-1278.
15.Kannel W, McGee D: Update on some epidemiologic features of intermittent claudication: the Framingham Study. Journal of the American Geriatrics Society 1985, 33(1):13
16.Selvin E, Erlinger TP: Prevalence of and risk factors for peripheral arterial disease in the united states results from the national health and nutrition examination survey, 1999–2000. Circulation 2004, 110(6):738-743.
17.Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA: Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. American journal of epidemiology 2001, 153(7):666-672.
18.Allison MA, Denenberg JO, Criqui MH: Family History of Peripheral Artery Disease Is Associated With Prevalence and Severity of Peripheral Artery Disease. Journal of the American College of Cardiology 2011, 58(13).
19.Valentine RJ, Guerra R, Stephan P, Scoggins E, Clagett GP, Cohen J: Family history is a major determinant of subclinical peripheral arterial disease in young adults. Journal of vascular surgery 2004, 39(2):351-356
20.Ridker PM, Stampfer MJ, Rifai N: Novel risk factors for systemic atherosclerosis. JAMA: the journal of the American Medical Association 2001, 285(19):2481-2485
21.Shammas NW (2007). "Epidemiology, classification, and modifiable risk factors of peripheral arterial disease". Vasc Health Risk Manag 3 (2): 229–34..
22.Selvin E, Wattanakit K, Steffes MW, Coresh J, Sharrett AR (April 2006). "HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study". Diabetes Care 29 (4): 877–82.
23.Gey DC, Lesho EP, Manngold J. Management of peripheral arterial disease. Am Fam Physician 2004;69:525–532.
24..Mannava K, Money SR. Current management of peripheral arterial occlusive disease: A review of pharmacologic agents and other interventions. Am J Cardio Drugs 2007;7(1);59–66.
25.TransAtlantic Inter-Society Consensus (TASC) Working Group. Management of peripheral arterial disease (PAD). TransAtlantic Inter- Society Consensus (TASC). Section B. intermittent claudication. Eur J Vasc Endovasc Surg 2000;19(Suppl A):47–114.
26.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003;289:2560– 2571.
27.National Cholesterol Education Program Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497.
28.Aronow WS. Management of peripheral arterial disease of the lower extremities in elderly patients. J Gerontol Series A Biol Sci Med Sci 2004;59:172–177.
29.Carman TL, Fernandez BB, Jr. A primary care approach to the patientwith claudication. Am Fam Physician 2000;61:1027–1032, 1034.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









