About Authors:
Aruna Rastogi
Roorkee College of Pharmacy and UTU
Patanjali Ayurved Ltd, Sr. Chemist
arunarastogi10@gmail.com
1. INTRODUCTION
1.1 NOVEL DRUG DELIVERY SYSTEM:
The method by which a drug is delivered can have a significant effect on its efficacy. Some drugs have an optimum concentration range within which maximum benefit is derived, and concentrations above or below this range can be toxic or produce no therapeutic benefit at all. On the other hand, the very slow progress in the efficacy of the treatment of severe diseases, has suggested a growing need for a multidisciplinary approach to the delivery of therapeutics to targets in tissues. From this, new ideas on controlling the pharmacokinetics, pharmacodynamics, non-specific toxicity, immunogenicity, biorecognition, and efficacy of drugs were generated. These new strategies, often called drug delivery systems (DDS), are based on interdisciplinary approaches that combine polymer science, pharmaceutics, bioconjugate chemistry, and molecular biology.
REFERENCE ID: PHARMATUTOR-ART-1652
To minimize drug degradation and loss, to prevent harmful side-effects and to increase drug bioavailability and the fraction of the drug accumulated in the required zone, various drug delivery and drug targeting systems are currently under development. Among drug carriers one can name soluble polymers, microparticles made of insoluble or biodegradable natural and synthetic polymers, microcapsules, cells, cell ghosts, lipoproteins, liposomes, and micelles. The carriers can be made slowly degradable, stimuli-reactive (e.g., pH- or temperature-sensitive), and even targeted (e.g., by conjugating them with specific antibodies against certain characteristic components of the area of interest). Targeting is the ability to direct the drug-loaded system to the site of interest. Two major mechanisms can be distinguished for addressing the desired sites for drug release: (i) passive and (ii) active targeting. An example of passive targeting is the preferential accumulation of chemotherapeutic agents in solid tumors as a result of the enhanced vascular permeability of tumor tissues compared with healthy tissue. A strategy that could allow active targeting involves the surface functionalization of drug carriers with ligands that are selectively recognized by receptors on the surface of the cells of interest. Since ligand–receptor interactions can be highly selective, this could allow a more precise targeting of the site of interest.
Controlled drug release and subsequent biodegradation are important for developing successful formulations. Potential release mechanisms involve: (i) desorption of surface-bound /adsorbed drugs; (ii) diffusion through the carrier matrix; (iii) diffusion (in the case of nanocapsules) through the carrier wall; (iv) carrier matrix erosion; and (v) a combined erosion /diffusion process. The mode of delivery can be the difference between a drug’s success and failure, as the choice of a drug is often influenced by the way the medicine is administered. Sustained (or continuous) release of a drug involves polymers that release the drug at a controlled rate due to diffusion out of the polymer or by degradation of the polymer over time. Pulsatile release is often the preferred method of drug delivery, as it closely mimics the way by which the body naturally produces hormones such as insulin. It is achieved by using drug-carrying polymers that respond to specific stimuli (e.g., exposure to light, changes in pH or temperature).
For over 20 years, researchers have appreciated the potential benefits of nanotechnology in providing vast improvements in drug delivery and drug targeting. Improving delivery techniques that minimize toxicity and improve efficacy offers great potential benefits to patients, and opens up new markets for pharmaceutical and drug delivery companies. Other approaches to drug delivery are focused on crossing particular physical barriers, such as the blood brain barrier, in order to better target the drug and improve its effectiveness; or on finding alternative and acceptable routes for the delivery of protein drugs other than via the gastro-intestinal tract, where degradation can occur.
1.1.1 Drug Delivery Carriers
Colloidal drug carrier systems such as micellar solutions, vesicle and liquid crystal dispersions, as well as nanoparticle dispersions consisting of small particles of 10–400 nm diameter show great promise as drug delivery systems. When developing these formulations, the goal is to obtain systems with optimized drug loading and release properties, long shelf-life and low toxicity. The incorporated drug participates in the microstructure of the system, and may even influence it due to molecular interactions, especially if the drug possesses amphiphilic and/or mesogenic properties.

Figure: 1 Pharmaceutical carriers
a) MICELLES:
Micelles formed by self-assembly of amphiphilic block copolymers (5-50 nm) in aqueous solutions are of great interest for drug delivery applications. The drugs can be physically entrapped in the core of block copolymer micelles and transported at concentrations that can exceed their intrinsic water- solubility. Moreover, the hydrophilic blocks can form hydrogen bonds with the aqueous surroundings and form a tight shell around the micellar core. As a result, the contents of the hydrophobic core are effectively protected against hydrolysis and enzymatic degradation. In addition, the corona may prevent recognition by the reticuloendothelial system and therefore preliminary elimination of the micelles from the bloodstream. A final feature that makes amphiphilic block copolymers attractive for drug delivery applications is the fact that their chemical composition, total molecular weight and block length ratios can be easily changed, which allows control of the size and morphology of the micelles. Functionalization of block copolymers with crosslinkable groups can increase the stability of the corresponding micelles and improve their temporal control. Substitution of block copolymer micelles with specific ligands is a very promising strategy to a broader range of sites of activity with a much higher selectivity.

Figure: 2 Block copolymer micelles.
b) LIPOSOMES:
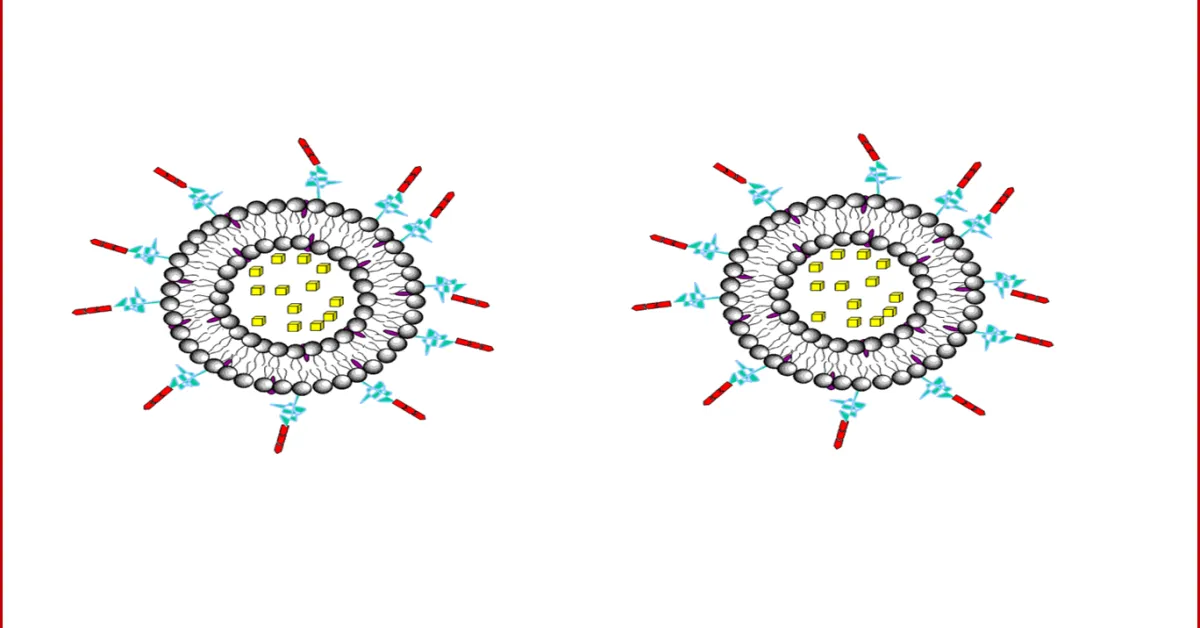
Liposomes are a form of vesicles that consist either of many, few or just one phospholipid bilayers. The polar character of the liposomal core enables polar drug molecules to be encapsulated. Amphiphilic and lipophilic molecules are solubilized within the phospholipid bilayer according to their affinity towards the phospholipids. Participation of nonionic surfactants instead of phospholipids in the bilayer formation results in niosomes. Channel proteins can be incorporated without loss of their activity within the hydrophobic domain of vesicle membranes, acting as a size-selective filter, only allowing passive diffusion of small solutes such as ions, nutrients and antibiotics. Thus, drugs that are encapsulated in a nanocage-functionalized with channel proteins are effectively protected from premature degradation by proteolytic enzymes. The drug molecule, however, is able to diffuse through the channel, driven by the concentration difference between the interior and the exterior of the nanocage.

Figure: 3 Drug encapsulation in liposomes
c) DENDRIMERS:
Dendrimers are nanometer-sized, highly branched and monodisperse macromolecules with symmetrical architecture. They consist of a central core, branching units and terminal functional groups. The core together with the internal units, determine the environment of the nanocavities and consequently their solubilizing properties, whereas the external groups the solubility and chemical behaviour of these polymers. Targeting effectiveness is affected by attaching targeting ligands at the external surface of dendrimers, while their stability and protection from the Mononuclear Phagocyte System (MPS) is being achieved by functionalization of the dendrimers with polyethylene glycol chains (PEG).
d) LIQUID CRYSTALS:
Liquid Crystals combine the properties of both liquid and solid states. They can be made to form different geometries, with alternative polar and non-polar layers (i.e., a lamellar phase) where aqueous drug solutions can be included.
d) LIQUID CRYSTALS:
Liquid Crystals combine the properties of both liquid and solid states. They can be made to form different geometries, with alternative polar and non-polar layers (i.e., a lamellar phase) where aqueous drug solutions can be included.
e) NANOPARTICLES:
Nanoparticles (including nanospheres and nanocapsules of size 10-200 nm) are in the solid state and are either amorphous or crystalline. They are able to adsorb and/or encapsulate a drug, thus protecting it against chemical and enzymatic degradation. Nanocapsules are vesicular systems in which the drug is confined to a cavity surrounded by a unique polymer membrane, while nanospheres are matrix systems in which the drug is physically and uniformly dispersed. Nanoparticles as drug carriers can be formed from both biodegradable polymers and non-biodegradable polymers. In recent years, biodegradable polymeric nanoparticles have attracted considerable attention as potential drug delivery devices in view of their applications in the controlled release of drugs, in targeting particular organs / tissues, as carriers of DNA in gene therapy, and in their ability to deliver proteins, peptides and genes through the peroral route.
f) HYDROGELS:
Hydrogels are three-dimensional, hydrophilic, polymeric networks capable of imbibing large amounts of water or biological fluids. The networks are composed of homopolymers or copolymers, and are insoluble due to the presence of chemical crosslinks (tie-points, junctions), or physical crosslinks, such as entanglements or crystallites. Hydrogels exhibit a thermodynamic compatibility with water, which allows them to swell in aqueous media. They are used to regulate drug release in reservoir-based, controlled release systems or as carriers in swellable and swelling-controlled release devices. On the forefront of controlled drug delivery, hydrogels as enviro-intelligent and stimuli-sensitive gel systems modulate release in response to pH, temperature, ionic strength, electric field, or specific analyte concentration differences. In these systems, release can be designed to occur within specific areas of the body (e.g., within a certain pH of the digestive tract) or also via specific sites (adhesive or cell-receptor specific gels via tethered chains from the hydrogel surface). Hydrogels as drug delivery systems can be very promising materials if combined with the technique of molecular imprinting.

Figure: 4 Pegylated and pH sensitive micro- or nanogels
1.1.2 Administration Routes
The choice of a delivery route is driven by patient acceptability, the properties of the drug (such as its solubility), access to a disease location, or effectiveness in dealing with the specific disease.
a) PERORAL ROUTE:
The most important drug delivery route is the peroral route. An increasing number of drugs are protein- and peptide-based. They offer the greatest potential for more effective therapeutics, but they do not easily cross mucosal surfaces and biological membranes; they are easily denatured or degraded, prone to rapid clearance in the liver and other body tissues and require precise dosing. At present, protein drugs are usually administered by injection, but this route is less pleasant and also poses problems of oscillating blood drug concentrations. So, despite the barriers to successful drug delivery that exist in the gastrointestinal tract (i.e., acid-induced hydrolysis in the stomach, enzymatic degradation throughout the gastrointestinal tract by several proteolytic enzymes, bacterial fermentation in the colon), the peroral route is still the most intensively investigated as it offers advantages of convenience and cheapness of administration, and potential manufacturing cost savings.
b) PULMONARY ROUTE:
Pulmonary delivery is also important and is effected in a variety of ways - via aerosols, metered dose inhaler systems (MDIs), powders (dry powder inhalers, DPIs) and solutions (nebulizers), all of which may contain nanostructures such as liposomes, micelles, nanoparticles and dendrimers. Aerosol products for pulmonary delivery comprise more than 30% of the global drug delivery market. Research into lung delivery is driven by the potential for successful protein and peptide drug delivery, and by the promise of an effective delivery mechanism for gene therapy (for example, in the treatment of cystic fibrosis), as well as the need to replace chlorofluorocarbon propellants in MDIs. Pulmonary drug delivery offers both local targeting for the treatment of respiratory diseases and increasingly appears to be a viable option for the delivery of drugs systemically. However, the pulmonary delivery of proteins suffers by proteases in the lung, which reduce the overall bioavailability, and by the barrier between capillary blood and alveolar air (air-blood barrier).
c) TRANSDERMAL ROUTE:
Transdermal drug delivery avoids problems such as gastrointestinal irritation, metabolism, variations in delivery rates and interference due to the presence of food. It is also suitable for unconscious patients. The technique is generally non-invasive and aesthetically acceptable, and can be used to provide local delivery over several days. Limitations include slow penetration rates, lack of dosage flexibility and / or precision, and a restriction to relatively low dosage drugs.
d) PARENTERAL ROUTE: Parenteral routes (intravenous, intramuscular, subcutaneous) are very important. The only nanosystems presently in the market (liposomes) are administered intravenously. Nanoscale drug carriers have a great potential for improving the delivery of drugs through nasal and sublingual routes, both of which avoid first-pass metabolism; and for difficult-access ocular, brain and intra-articular cavities. For example, it has been possible to deliver peptides and vaccines systemically, using the nasal route, thanks to the association of the active drug macromolecules with nanoparticles. In addition, there is the possibility of improving the occular bioavailability of drugs if administered in a colloidal drug carrier.
e) TRANS TISSUE AND LOCAL ROUTE:
Trans-tissue and local delivery systems require to be tightly fixed to resected tissues during surgery. The aim is to produce an elevated pharmacological effect, while minimizing systemic, administration-associated toxicity. Trans-tissue systems include: drug-loaded gelatinous gels, which are formed in-situ and adhere to resected tissues, releasing drugs, proteins or gene-encoding adenoviruses; antibody-fixed gelatinous gels (cytokine barrier) that form a barrier, which, on a target tissue could prevent the permeation of cytokines into that tissue; cell-based delivery, which involves a gene-transduced oral mucosal epithelial cell (OMEC)-implanted sheet; device-directed delivery - a rechargeable drug infusion device that can be attached to the resected site.
f) GENE DELIVERY:
Gene delivery is a challenging task in the treatment of genetic disorders. In the case of gene delivery, the plasmid DNA has to be introduced into the target cells, which should get transcribed and the genetic information should ultimately be translated into the corresponding protein. To achieve this goal, a number of hurdles are to be overcome by the gene delivery system. Transfection is affected by: (a) targeting the delivery system to the target cell, (b) transport through the cell membrane, (c) uptake and degradation in the endolysosomes and (d) intracellular trafficking of plasmid DNA to the nucleus.
2. NANOPARTICLES
The prefix nano means a billionth (10-9). Thus, a nanometre (nm) is a billionth of a metre. Nanotechnology is concerned with the creation or manipulation of particles and materials whose minimum dimensions are nanometric, though normally less than 100 nm. These materials may be produced from the structured organization of groups of atoms and molecules or by reducing macroscopic materials to a nanometric scale.
The current definition defines nanoparticles as “particles with at least one dimension smaller than 100 nm or 0.1 μm, and with different properties than particles of larger diameters made of the same material”.
While the development of nanotechnologies is a very modern, multidisciplinary science, the manufacture of nanomaterials, both by nature and by humans, dates from time immemorial.Indeed, several natural structures, including proteins and the DNA diameter fit the above definition of nanomaterials.
while viruses represent the smallest naturally occurring functional nano-objects. To illustrate the orders of magnitude involved, the diameter of a DNA molecule is of the order of 2 to 12 nanometres (nm), a red blood cell has a diameter of 5,000 nm and a human hair a diameter 10,000 to 50,000 nm.
Romans in the pre-Christian era were already introducing metals with nanometric dimensions in glass-making; a cup describing the death of King Lycurgus (circa 800 BC) contains nanoparticles of silver and gold; when a light source is placed inside the cup, its colour changes from green to red.
The colours of certain Mayan paintings stem from the presence of metallic nanoparticles, as does the lustre of Italian Renaissance pottery. The stained-glass windows of the great medieval cathedrals also contain metallic nanoparticles.Photography, which was developed in the 18th and 19th centuries, provides a more recent example of the use of nanoparticles, which in this example is made up of particles of silver sensitive to light.
Due to their low granulometry, many condensation products deriving from the combustion process contain nanoparticles; these include diesel gases, industrial furnace emissions and welding fumes. In 1993 alone, synthesis through flame pyrolysis of six million tonnes of carbon black with a high specific surface area produced carbon powder of nanometric dimensions. Combustion or flame pyrolysis is also used in the mass production of silica fume, ultrafine titanium dioxide particles and ultrafine metal particles, all of nanometric dimensions.
In addition, the definition of nanoparticles based on size, allows us to include colloids and soils that have been used for over a hundred years. In 1857, Faraday, had already described the use of colloidal gold in his experiments . Since then, colloidal science has evolved a lot. The new colloids are used in the production of metals, oxides and organic and pharmaceutical products. Given this broad definition, it is important to home in on the subject-matter of our inquiry.
In 1960, Richard Feynman, the 1965 Nobel prizewinner in Physics, began speculating on the possibilities and potential of nanometric materials, and on the fact that the manipulation of individual atoms could allow us to create very small structures whose properties would be very different from larger structures with the same composition. With the major technological developments of recent decades, it has now become possible to manipulate atoms one by one. It has been demonstrated that these structures do, in effect, have unique properties, which accounts for the interest in research in this field, especially over the last decade.
Articles describing nanomaterials may be divided into two major categories:
a)those that are produced by collecting individual atoms; this is the bottom-up approach, and
b)those that are produced by subdividing bulk materials into nanometric sizes; this is the top-down approach.
In both cases, their dimensions are smaller than the critical length characterizing most physical phenomena, and this is what gives them their unique properties.
Nanomaterials often demonstrate characteristics such as extraordinary strength or unsuspected electrical, physical or chemical properties that are completely different from those demonstrated by the same products with larger dimensions.
The fields with current commercial uses and producing the greatest revenue are mechanico-chemical polishing, magnetic recording tapes, sunscreens, automotive catalyst supports, bio-labeling, electroconductive coatings and optical fibers. The biomedical and pharmaceutical fields, electronics, metallurgy, agriculture, textiles,coatings, cosmetics, energy and catalysts are other sectors with growing applications. Roco (2004) maintains that we are already in the second generation of the nanotechnology age.
The first generation dealt with passive nanostructures such as coatings, nanoparticles, nanostructured metals, polymers and ceramics.
The current generation deals with active nanostructures such as transistors, amplifiers, targeteddrugs and adaptive structures.
Many observers think that nanoparticles and nanotechnologies will constitute the focus of the next industrial revolution. Research in the field is growing very rapidly and all industrialized countries see potential for expansion and applications in numerous fields as well as colossal potential economic spin-offs. Governments and large companies are developing strategies and investing massively in research. For example, Europe has made nanotechnology one of its seven priority project-oriented research areas and is investing 1.3 billion Euros in it for the 2002-2006 period. In the United States, the National Nanotechnology Initiative (NNI) budget for 2005 alone amounted to a billion dollars.
The Government of Canada is currently building a research centre in Alberta that will be dedicated exclusively to nanotechnologies. The federal government is also preparing a national nanotechnology plan. Quebec has set up NanoQuebec to support the transfer and marketing of applications developed in universities, and to increase the use of nanotechnologies in research on problems encountered by Quebec companies in all industrial sectors.
Unfortunately, only a very small proportion of research on nanoparticles is concerned with its occupational health and safety risks, or with its threat to the environment and the health of populations. The field of nanomaterials and nanotechnologies cannot be covered exhaustively because it is too vast, too multidisciplinary and is changing too rapidly. Nevertheless, the present report, which is based on a literature review extending to June 2005, is designed to provide an overall portrait of nanomaterials and nanotechnologies as well as their main potential applications.
It places special emphasis on the situation in Quebec, the known risks to the health and safety of workers and prevention. Rapid technological changes have already facilitated the start-up of about forty nanotechnology companies in Quebec. Further efforts must be made in the area of health and safety know-how transfer to effectively support Quebec companies and research teams investigating ways to protect the health and safety of workers producing or using these substances.
ADVANTAGES:
* Increased efficacy and therapeutic index.
* Increased stability via encapsulation.
* Improved pharmacokinetic effect.
* Producible with various sizes,compound surface propt’s.
* Entrap both hydrophilic & lipophillic drug Protect entrapped drug from enzymatic degradation.
* Large variety of drugs(antineoplastic, antibiotic) peptides or protein(including antibodies) &viruses &bacteria can be incorporated into nanoparticles.
* Water soluble drugs are trapped in aqueous compartment & lipophillic drugs without the need for chemical modification.
* Nanoparticles encapsulated drugs are delivered intact to various tissue and cells and can be released when nanoparticles are destroyed ,enabling site specific and targeted drug delivery.
* Other tissues and cells of the body are protected from drug until it is released by nanoparticles thus decreasing drug toxicity.
* Size change and other characteristics can be altered depending on the rug and intended use of the product.
DISADVANTAGES:
* Include their tendency to be taken up by cells of reticoendothelial system and the slow release of the drug when the liposomes are taken up by phagocytes through endocytosis,fusion,surface adsorption or lipid exchange.
* Stabilizing the formulated liposomes is also difficult, but many approaches are now used for their stabilization.
2.1 Classification:
Briefly, nanomaterials can be classified in terms of dimensioning of the nanostructures involved
A) one nanometric dimension: surface coatings, thin films and interfaces.
B) Two nanometric dimensions: nanometric domain. Nanotubes, dendrimers, nanowires, fibers and fibrils.
C) Three nanometric dimentions: quantum dots or nanocrystals, fullerenes, particles, precipitates, colloids and catalysts.
One-dimensional systems, such as thin films or manufactured surfaces, have been used for decades in electronics, chemistry and engineering. Production of thin films or monolayers is now commonplace in the electronic field, just as the use of customized surfaces is common in the field of solar cells or catalysis. These fields are well known and the risks are properly controlled.
The properties of two-dimensional systems (carbon nanotubes, inorganic nanotubes, nanowires and biopolymers) are less understood and the manufacturing capabilities are less advanced.
Finally, some 3-D systems, such as natural nanomaterials and combustion products, metallic oxides, carbon black, titanium oxide (TiO2) and zinc oxide (ZnO) are well known, while others such as fullerenes, dendrimers and quantum dots represent the greatest challenges in terms of production and understanding of properties (Royal Society and Royal Academy of Engineering, 2004).
2.2 Characteristics and Properties of Nanoparticles:
Nanoparticles display properties that differ from those of the bulk materials from which they derive. In general, the integration of nanoparticles will seek modification of electrical, mechanical, magnetic, optical or chemical properties. Here are the main examples:
A) Fullerenes
Fullerenes are spherical cages containing from 28 to more than 100 carbon atoms . The most widely studied form, synthesized for the first time in 1985, contains 60 carbon atoms,(C60). This is a hollow ball composed of interconnected carbon pentagons and hexagons, resembling a soccer ball. Fullerenes are a class of materials displaying unique physical properties. They can be subjected to extreme pressures and regain their original shape when the pressure is released.
These molecules do not combine with each other, thus giving them major potential for application as lubricants. When fullerenes are manufactured, certain carbon atoms can be replaced with nitrogen atoms and form bondable molecules, thus producing a hard but elastic material. Fullerenes, whether modified or not, have also shown major potential as catalysts. They have interesting electrical properties and it has been suggested to use them in the electronics field, ranging from data storage to production of solar cells.

Figure: 5 Schematic representation of a fullerene
Incorporating them into carbon nanotubes modifies the electrical behaviour of fullerenes, creating regions with varying semiconductive properties, thus offering potential applications in nanoelectronics. Their properties vary according to wavelength, thus finding applications in telecommunications. Since fullerenes are empty structures with dimensions similar to several biologically active molecules, they can be filled with different substances and find medical applications.

Figure: 6 Schematic representation of a modified fullerene
B) Carbon nanotubes:
Discovered barely a decade ago, carbon nanotubes are a new form of carbon molecule. Wound in a hexagonal network of carbon atoms, these hollow cylinders can have diameters as small as 0.7 nm and reach several millimeters in length. Each end can be opened or closed by a fullerene half-molecule. These nanotubes can have a single layer (like a straw) or several layers (like a poster rolled in a tube) of coaxial cylinders of increasing diameters in a common axis. Multilayer carbon nanotubes can reach diameters of 20 nm.
The small dimensions of carbon nanotubes, combined with their remarkable physical, mechanical and electrical properties, make them a unique material. They display metallic or semiconductive properties, depending onhow the carbon leaf is wound on itself. The current density that a nanotube can carry is extremely high and can reach one billion amperes per square metre, making it a superconductor. Light and flexible, the mechanical strength of carbon nanotubes is more than sixty times greater than that of the best steels, even though they weigh six times less. They also present a very large specific surface area, are excellent heat conductors , and display unique electronic properties, offering a threedimensional configuration. They have a great capacity for molecular absorption. Moreover, they are chemically and thermally very stable.

Figure: 7 Schematic representation ofmonolayer or multilayer carbon nanotubes or nanotubes containing other elements
C) Nanowires
Nanowires are conductive or semiconductive particles with a crystalline structure of a few dozen nm and a high length/diameter ratio. Silicon, cobalt, gold or copper-based nanowires have already been produced. They are used to transport electrons in nanoelectronics. They could be composed of different metals, oxides, sulphides and nitrides.
D) Carbon nanofoams
Carbon nanofoams are the fifth known allotrope of carbon, after graphite, diamond, carbon nanofibers and fullerenes. In carbon nanofoam, islands of carbon atoms, typically from 6 to 9 nm, are randomly interconnected to form a very light, solid and spongy three-dimensional structure, which can act as a semiconductor. Carbon nanofoams display temporary magnetic properties.
E) Quantum dots
An important field of research for about the past five years, quantum dots (also called nanocrystals or artificial atoms) represent a special form of spherical nanocrystals from 1 to 10 nm in diameter. They have been developed in the form of semiconductors, insulators, metals, magnetic materials or metallic oxides. The number of atoms in quantum dots, which can range from 1,000 to 100,000, makes them neither an extended solid structure nor a molecular entity. The principal research studies have focused on semiconductor quantum dots, which display distinctive quantal effects depending on the dimensions. The light emitted can be adjusted to the desired wavelength by changing the overall dimension.

Figure: 8 Different forms of quantum dots showthe organization of the individual atoms
F) Dendrimers
Dendrimers represent a new class of controlled-structure polymers with nanometric dimensions. They are considered to be basic elements for large-scale synthesis of organic and inorganic nanostructures with dimensions of 1 to 100 nm, displaying unique properties. Dendrimers allow precise, atom-by-atom control of the synthesis of nanostructures according to the desired dimensions, shape and surface chemistry. Given that dendrimers can be developed to display hydrophilic or hydrophobic characteristics, their uses can be highly diversified. With different reactive surface groupings, their abundant use is particularly envisioned in the medical and biomedical field. Compatible with organic structures such as DNA, they can also be fabricated to interact with metallic nanocrystals and nanotubes or to possess an encapsulation capacity or display a unimolecular functionality.
G) Other nanoparticles
Some nanoparticles tend to agglomerate and form structures in chains or with multiple branches. This category normally includes welding fumes, silica fumes, carbon black and other nanoparticles, which are often synthesized by flame pyrolysis. These nanoparticles may include metals, metallic oxides, semiconductors, ceramics and organic material. They may also include composites with a metallic core and an oxide or alloy coating, for example. Colloids, which have been known for a long time, are nanometric dimensions. These nanoparticles will not be considered in this study.
2.3 Nanoparticle characterization tools
The characterization of nanomaterials and the understanding of their behaviour is fundamental to the development of new applications and the reproducible and reliable production of nanomaterials. Process nanometrology uses precision instruments with very high sensitivity, capable of measuring at dimensions that are often less than a nanometer. These instruments allow manipulation of individual atoms and measurement of lengths, shapes, forces, masses, electrical properties and other physical properties. They also use electron beam techniques, including high-resolution transmission electron microscopy. Scanning probe techniques include scanning tunneling microscopy and atomic force microscopy. Optical manipulators allow manipulation and measurement of individual atoms.
3. DEVELOPMENT AND PRODUCTION OF NANOPARTICLES
Development of nanoparticles and nanotechnologies is currently one of the most active research fields worldwide. Several industrialized countries are making it a strategic priority for sustainable technological, economic and societal development. Indeed, in 2001 it was estimated that the potential world market would reach one thousand billion1 US dollars by 2015. In 2003, the British Department of Trade and Industry estimated that there would be a world market of USD100 billion by 2005 (Arnall, 2003). The creation of the US Nanobusiness Alliance, the Europe Nanobusiness Association and the Asia-Pacific Nanotechnology Forum, whose shared objective is to commercialize nanoproducts, clearly illustrates the expected magnitude of these markets and the international competition in the field. Quebec is doing likewise via NanoQuebec.
3.1 Worldwide research efforts
A study published in 2002 concluded that, from 1989 to 1998, the rate of increase of scientific publications on nanomaterials increased annually by 27%. This data indicated that over 30 countries were involved in research in this field, the most active being the United States, Japan, China, France, Great Britain and Russia, which accounted for 70% of publications. Also in 2002, Holister concluded that 455 private companies and 271 academic institutions and government entities were already involved in researching short-term applications in nanotechnology around the world. Since then, this field has continued to grow.
Over the past five years, many countries have developed strategic plans and decided to invest massively in nanotechnology research. This will result in an ongoing increase in scientific articles on the subject and a wider variety of research topics. Roco (2001, 2003) reported that government investments had risen from USD 432 million in 1997 to over USD 2.98 billion in 2003.
Worldwide research efforts are currently estimated at over USD 8 billion for the year 2005 alone, about 40% of which would come from the private sector. A detailed review of international investments was carried out by Waters (2003) and by the European Commission (2004a). Despite these colossal investments aimed at development of new commercial applications, research in the occupational health and safety field is still in its infancy.
Five leading Asian countries are heavily involved in research into the development of new products: Japan, China, South Korea, Taiwan and Singapore. Japan is the most important Asian stakeholder in the field and has a completely integrated development policy, which the government sees as the key to the country’s economic recovery. In 2003, the Japanese government invested the equivalent of USD 800 million in the nanotechnology field, while the private sector invested an additional USD 830 million. The British Department of Trade and Industry reported, in 2002, that the first 1 One thousand billion or one million million carbon nanotube and fullerene production plants were under construction in Japan.
The European Union’s 6th Framework Program, known as Nanoforum, allocated USD1.44 billion for the 2002-2006 period and is seeking to develop a European research and communications network integrating all aspects of nanotechnology, ranging from business to science and information intended for the general public. In May 2004, the Commission adopted a plan in which it proposed a safe, integrated and responsible European strategy. Following broad consultations of its members, the 7th Framework Program , proposes to increase the European Union’s R&D investments to strengthen Europe’s global position in this field.
One of the specific objectives of this European initiative is long-term interdisciplinary research to understand the phenomena involved, master the processes and develop research tools. There is particular interest in nanobiotechnologies, nanoengineering techniques, implications for the fields of health and medical systems, chemistry, energy, optics, food and the environment. This European program also covers production and processing of multifunctional materials, the involvement of engineering for the development of materials, and the development of new processes and flexible and intelligent manufacturing systems. The specific initiatives of several countries must be added to these European efforts. Waters (2003) estimates that the aggregate European investments will range between dollar 3.8 billion and dollar 7.8 billion in 2002-2006. However, private enterprise seems to be much less active than in the United States or Japan. Among the most active European countries are Germany, Great Britain, France, Switzerland, Belgium and the Netherlands. Other European countries are also active in nanotechnology R&D, but their investments are more limited: Ireland, Luxembourg, Italy, Austria, Denmark, Finland, Sweden and Norway.
3.2 The most actively studied nanoparticles
The most active research in the nanoparticle field concerns carbon nanotubes, which are expected to have a wide variety of applications in numerous fields. In particular, the use of nanotubes is being considered in electronics, in electrochemistry, as mechanical reinforcements for high-performance composites, as cathode ray transmitters, as a means of energy production or hydrogen storage, or as templates for the creation of other nanostructures, such as production of metallic nanowires by filling carbon tubes. The exceptional strength of the bonds uniting the carbon atoms in a nanotube structure makes them an ideal candidate as reinforcing agents in composites. Among the other uses envisioned, carbon nanotubes could be employed as sensors for high-resolution imaging, in nanolithography, in production of nanoelectrodes or as vectors to transport drugs to specific locations in the human body.
3.3 Polymers used in nanoparticles
Polymeric Nanoparticles:
As name only suggest polymeric nanoparticles are nanoparticles which are
prepared from polymers. The drug is dissolved, entrapped, encapsulated or
attached to a nanoparticles and depending upon the method of preparation,
nanoparticles, nanospheres or nanocapsules can be obtained. Nanocapsules
are vesicular systems in which the drug is confined to a cavity surrounded by
a polymer membrane, while nanospheres are matrix systems in which the
drug is physically and uniformly dispersed.
In recent years, biodegradable polymeric nanoparticles have attracted
considerable attention as potential drug delivery devices in view of their
applications in drug targeting to particular organs/tissues, as carriers of DNA
in gene therapy, and in their ability to deliver proteins, peptides and genes
through a per oral route of administration.
Classification: Polymers are classified as:
A) Natural polymers:
· Gums (Ex. Acacia, Guar, etc.)
· Chitosan
· Gelatin
· Sodium alginate
· Albumin
B) Synthetic polymers:
a) Nonbiodegradable:
· Cellulosics.
· Poly(2-hydroxy ethyl methacrylate).
· Poly(N-vinyl pyrrolidone).
· Poly(methyl methacrylate).
· Poly(vinyl alcohol).
· Poly(acrylic acid).
· Polyacrylamide.
· Poly(ethylene-co-vinyl acetate).
· Poly(ethylene glycol).
· Poly(methacrylic acid).
b) Biodegradable
· Polylactides (PLA).
· Polyglycolides (PGA).
· Poly(lactide-co-glycolides) (PLGA).
· Polyanhydrides.
· Polyorthoesters.
· Polycyanoacrylates
· Polycaprolactone
Originally, polylactides and polyglycolides were used as absorbable suture material. The main advantage of these degradable polymers is that they are broken down into biologically acceptable molecules that are metabolized and removed from the body via normal metabolic pathways. However, biodegradable materials do produce degradation by-products that must be
tolerated with little or no adverse reactions within the biological environment.
Characteristic features of polymers: A polymer used in controlled drug delivery formulations, must be:
- Chemically inert
- Non-toxic
- Free of leachable impurities
- An appropriate physical structure
- With minimal undesired aging
- Readily processable
Abraxane is the first polymeric nanoparticle based product from American Pharmaceutical Partners, Inc., and American Bioscience, Inc. (ABI). It was approved in year 2005 and is consisting of albumin-bound paclitaxel nanoparticles. This product is free of toxic solvents like cremophor-EL, M which is used until now to solubalize paclitaxel in order to administer it
intravenously to the patient. cremophor-EL is known to cause life-threatening allergic reactions. Success of Abraxane show that nanotechnology can bring many exciting products which can overcome many hurdle of formulation scientist.
3.4 PREPARATION TECHNIQUES OF NANOPARTICLES:
The selection of appropriate method for the preparation of nanoparticles depend on the physicochemical characteristics of the polymer and the drug to be loaded. On the contrary, the preparation techniques largely determine the inner structure, in vitro release profile and biological fate of these polymeric delivery systems. Two type of system with different inner structure are apparently possible including:
· MATRIX TYPE SYSTEM: This consisting of an entanglement of oligomer or polymer units (nanoparticles / nanospheres ).
· RESERVOIR TYPE SYSTEM: This comprised of an oily core surrounded by an embryonic polymeric shell(nanocapsules).
The drug can either be entrapped within the reservoir or the matrix or otherwise be absorbed on the surface of these particulate systems. The polymers are strictly structured to a nanometric size range particle using appropriate methodologies. These methodologies are conveniently classified as follows:
I. AMPHIPHILIC MACROMOLECULE CROSS-LINKING:
a) Heat cross-linking
b) Chemical cross-linking
c) Emulsion chemical dehydration
d) Phase separation in aqueous medium (Desolvation)
e) PH-induced aggregation
f) Counter ion induced aggregation
II. Polymerization based methods:
a) Polymerization of monomers
b) Emulsion (micellar) polymerization
1. Conventional emulsion polymerization
· Micellar nucleation and polymerisation
· Homogenous nucleation and polymerization
2. Inverse emulsion polymerization
c) Dispersion polymerization
d) Interfacial condensation polymerization
e) Interfacial complexation
III. POLYMER PRECIPITATION MEYHODS
a) Solvent extraction /evaporation
b) Solvent displacement(Nanoprecipitation)
c) Salting out
AMPHIPHILIC MACROMOLECULE CROSS-LINKING:
The macromolecules used in the preparation are amphiphilic in nature means having capacity to contain both lipophilic and hydrophilic drugs. The preparation by this method involve the aggregation of amphiphiles and then cross-linking by heat or chemical cross-linking.
a) Heat cross-linking
b) Chemicalcross-linking
PROCEDURE:
NANOSPHERE PREPARATION BY CROSS LINKING OF AMPHIPHILIC MACROMOLECULES

Figure: 9 Heat denaturation process

Figure: 10 Chemical cross linking process

Figure: 11 Emulsion chemical dehydration process
(D) Phase separation in aquous medium:
The protein and polysaccharide from an aqueous phase Can be desolvated by PH
change, or change in temperature or by adding some appropriate counter ion.
PROCEDURE: It involves three steps:
Step1: Proteine dissolution
Step2: Proteine aggregation
Step3: Proteine deaggregation

Figure: 12 Phase separation in aquous medium process
NANOPARTICLE PREPARATION USING POLYMERIZATION BASED
Two methods are generally used:
1) Emulsion (micellar) polymerization: In which the monomer to be polymerized is emulsified in a non solvent phase.
GENERAL PROCEDURE OF EMULSIFICATION POLYMERISATION:
Step1: Monomer is emulsified in a non-solvent phase with surfactant molecule,then a monomer swollen micelle form
Step2: After the formation of micelles, polymerization takes place inside this. The two main process during polymerization are nucleation & propagation.
Step3: Reaction stop with the full consumption of the monomer & surfactant
Step4: We can add drug during the preparation or after the preparation.
If we add the drug in between the preparation,thy get encapsulated or dispersed in the matrix & if the drug is added after the preparation of the nanoparticles then they getadsorb to the surface of the nsanoparticles.
A) Conventional emulsion polymerization(O/W type): The emulsion is O/W type. The monomer is emulsified with the help of surfactant.
a) Micellar nucleation and polymerization (used when monomer is slightly soluble in outer phase).

Figure: 13 Micellar nucleation and polymerization process
b) Homogenous nucleation and polymerization (used when monomer is sufficiently soluble in outer phase)

Figure: 14 Homogenous nucleation and polymerization process
B) Inverse emulsion polymerization(W/O type)

Figure: 15 Inverse emulsion polymerization process
2) Dispersion polymerization: In which the monomer to be polymerized is emulsified in a solvent.

Figure: 16 Dispersion polymerization process
NANOPARTICLE PREPARATION USING POLYMER PRECIPITATION METHODS:
1)Double emulsion solvent evaporation:

Figure: 17Double emulsion solvent evaporation process

Figure: 18 Solvent displacement process

Figure: 19 Salting out process
4. EVALUATION OF NANOPARTICLES:
The nanoparticles are generally evaluated for the following:
1) Size and morphology
2) Specific surface
3) Surface charge and electrophoretic mobility
4) Density of nanoparticles
5) Molecular weight
6) Nanoparticle recovery and drug incorporation efficiency
7) In vitro release
1) Size and morphology:
Size of nanoparticle is determined by
a) Photon correlation spectroscopy(PCM)
b) Electron microscopy(EM): This include
1) Scaning electron microscopy(SEM)
2) Transmission electron microscopy(TEM)
3) Freez fraction electron microscopy(FFEM)
c) Atomic force microscopy
Electron microcopy is less time consuming, FFEM give the internel morphology of particles.
TEM and FFEM provide differentiation among nanoparticle and nanocapsules and emulsion droplets.
All the particles are coated with gold as they are non-conductive (thickness of gold coat is 30-50nm). Thus determined size should be denoted as gold-coated particle size.
Atomic force microscopy(AFM) is an advanced nanoscopic technique and used for characterization of PLA nanosphere.
Mercury porositometery is use to measure size of nanoparticles.
2) Specific surface:
Specific surface of freez dried nanoparticles is determined with sorptometer and it is calculated by using the formula:
A= 6/ D.d
Where
A= Specific surface
D= Density
d= Diameter of the particle
3) Surface charge and electrophoretic mobility:
Surface charge of nanoparticles can be determined by measuring the velocity of particle in an electronic field. It can also be measured as electrophoretic mobility.
The electrophoretic mobility is determined in phosphate saline buffer & human serum.
Laser Doppler anemometry or velocimetry is widely used techniques for determination of velocities.
Phosphate saline buffer (PH-7.4) reduces the charge value of nanoparticles, zeta potential can be obtained by measuring the electrophoretic mobility applying Helmholtz-Smoluchowski equation.
4) Density of nanoparticles:
Density of nanoparticles is determined with helum or air using a gas pychnometer
5) Molecular weight:
Molecular weight of polymer and its distribution in the matrix can be evaluated by Gel permeation chromatography.
6) Nanoparticle recovery and drug incorporation efficiency:
Nanoparticle recovery or Nanoparticle yield can be calculated using equation:

Drug incorporation efficiency or drug content can be calculated using equation:

7) In vitro release:
In vitro release is determined by using dialysis diffusion cell or nodified ultrafiltration technique and phosphate buffer is used for it.
Donar and receptor chambers are separated by milipore membrane. Donar compartment have nanoparticles along with phosphate buffer, while the receptor compartment contain only buffer solution.The receptor compartment is assayed for drug release at various time interval.
Devices used for evaluation:
The quantitative determination of exposure to nanoparticles currently poses a major challenge, because every environment already contains airborne particles in suspension. Through this mixture of dusts of different granulometries and various compositions, the content and characterization of nanoparticles must be determined. In industrial hygiene, airborne dust quantities are normally determined in units of mass per volume for most dusts, except in the case of fibres, which are counted.
These different ways of evaluating airborne dusts account for parameters improved the definition of the correlation between the health impairment risk and the exposure level.
The worker’s exposure measurements normally are taken in the respiratory zone. Because of the rapid major variances in particulate concentration observed for nanoparticles and ultrafine particles, the direct reading devices used must react quickly. Unfortunately, no device is currently adapted to sample such particles in the respiratory zone. The existing devices capable of detecting nanoparticles and ultrafine particles are bulky and not conducive to sampling in the work environment, even at fixed stations. Major developments will be required to determine the precise occupational exposure on a routine basis with user-friendly, robust and affordable instruments.
Nanoparticles can be detected by electrostatic methods, by condensation on particles to grow them until they are measurable optically, or by other methods. Electrostatic methods require that the particles be charged and are not very sensitive, while condensation on particles is the only means allowing detection of neutral aerosol particles, which are too small to be measured by the optical method. The existing devices using condensation techniques can cover a wide range of diameters and concentrations.
The devices using the detection methods described above are often used with other devices, such as electrical mobility analyzers acting as preselectors. Combining these two types of devices obtains the granulometries or fine structures of ultrafine aerosols. Most of the devices used for nanoparticle detection cannot discriminate between nanoparticle agglomerates or single particles. This is why they are limitative in terms of assessment of aerosol toxicity, because it has been shown that nanoparticle agglomerates can have increased toxicity compared to solid particles of the same size.
A) Devices for direct measurement of concentration
Different devices and techniques allow measurement of certain parameters of particles in suspension in a liquid or gaseous medium. These parameters generally are the particulate concentration by mass, surface or number present in a certain liquid or gaseous volume. Some devices or techniques also make it possible to obtain the granulometric structure of these suspensions, that is, obtain these parameters for the different particle sizes present.
Table briefly illustrates some of these devices or techniques and their range of applicability. The following sections briefly describe the relevant nanoparticle detection techniques.

Table: 1 Techniques available for determination of size analysis
B) Diffusion batteries
Diffusion batteries are used as preselectors for detection devices, such as condensation nucleuscounters (CNC). They use the well-known phenomenon of the increase in particle diffusioncapacity in inverse proportion to the decrease in particle diameter. Small particles passingthrough a sieve will interact with it more due to surface attraction forces and will be depositedmore quickly on the neighbouring walls than coarse particles. The batteries use this phenomenonto separate the particles by granulometry brackets. This separation technique can be used forparticles ranging from 2 nm to 200 nm but is most often applied for particlessmaller than 100 nm. Granulometric resolution is limited,which explains why the devices described above, using electrostatic classification, are preferred to diffusion batteries. TSI is the only manufacturer to offer such a device with its model 3040/3041.
C) Condensation nucleus counters (CNC)
Condensation nucleus counters (CNC) serve to measure the numerical concentrations of particles. They are also called condensation particle counters (CPC) or Aitken nucleus counters (ANC). This technique is applicable to the detection of particles ranging from 2 nm to 3000 nm. Their generally slow response time means that the number of concentration peaks is underestimated. However, their integrated values remain reliable. Table 2 lists some commercially available devices and their range of use.
Table: 2 Commercial condensation nucleus counter
|
Manufacturer |
Device |
Analytical range (nm) |
|
BGI |
Pollack counter |
>2.8 (1) |
|
Environmental One |
Rich 200 |
|
|
Gardner |
CNC |
>3.8 (1) |
|
TSI |
CPC 3007 (in real time) |
10 to >1000 |
|
TSI |
CPC 3022 |
> 6 (2) |
|
TSI |
UCPC 3025 |
> 3 (2) |
|
TSI |
P-Trak 8525 (in real time) |
20 to > 1000 |
|
TSI |
CNC, model 3760 |
> 14 (1) |
|
MET |
CNC, model 1100 |
> 10 (1) |
|
KAN |
CNC, model 3851 |
> 10 (1) |
|
ACT |
CNC, model 5000 |
> 50 (1) |
D) Electrical mobility analysers
Devices using electrical mobility make it possible to classify particles or deposit them onsurfaces. They use the drift of a charged particle in an electrical field to classify or deposit it. Electrical aerosol analyzers (EAA) and differential mobility analyzers (DMA) classify particles. Electrostatic precipitators (ESP) allow deposition for subsequent laboratory analysis, by microscopy, for example. Apart from electrostatic precipitators, these devices must be used in series with different detection devices to determine the granulometry of an ultrafine or nanofine aerosol. Thus, an electrostatic precipitator or an electrical aerosol sampler (EAS) must be combined with an electrometer, while a differential mobility analyzer (DMA) must be used with a condensation nucleus counter or an electrometer to obtain a granulometry. All these devices require precisely controlled sampling of the aerosol to be analyzed before the mobility analyses and their conversion into granulometry measurements. These techniques are difficult to apply for particles smaller than 10 nm because these particles are difficult to charge. The scanning mobility particle sizer (SMPS) and the differential mobility particle sizer (DMPS) are very bulky and barely portable. However, they rank among the best devices available for analysis of particles between 5 and 800 nm or between 8 and 200 nm. The SMPS is an EAA combined with a CNC, while the DMPS is a DMA connected in series with a CNC. The main devices and techniques and their range of application are listed in Table 3.
Table: 3 Main electrical mobility analysers
|
Manufacturer |
Device or technique |
Analytical range (nm) |
|
|
SMPS |
5 to 800 (1) |
|
In Tox Products |
ESP (with an electrometer) |
> 30 (2) |
|
Hauke KG |
Hauke-Aeras(SAAS3/150) |
3 to 150 (2) |
|
University of Vienna |
EMS VIE-06(requires a CNC) |
|
|
TSI |
SMPS model 3071 |
10 to 350 (3) |
|
|
MAS |
< 100 |
|
TSI |
EAS model 3100 |
< 500 |
|
TSI |
DMPS model 3092 |
10 to 1000 (4) |
|
TSI |
DMPS model 3932 |
10 to 1000 (2) |
E) Cascading impactors
Impactors allow separation of aerosol particles into different granulometry brackets. Each granulometry bracket is associated with a stage of the impactor, on which a collection substrate or a detection element is placed. Impactors offer the advantage of allowing subsequent analysis of particles on the collection substrates and gravimetric determinations. In general, the most common impactors have a range of use between 50 and 30,000 nm.
A wide range of impactors exist but some have been developed specifically for ultrafine particles. They have a lower detection limit. Among them, the micro-orifice uniform deposition impactor (MOUDI), model 110 can classify particles from diameters of 56 nm to 15000 nm while the nano-MOUDI allows three-stage collection of 32, 18 and 10 nm nanoparticles. Table 4 presents some commercial impactors usable for nanoparticles.
Table: 4 Some commercial impactors usable for nanoparticles
|
Manufacturer |
Device |
Analytical range (nm) |
|
Atmospheric Technology |
Low pressure impactor |
50-4000 (1) |
|
Andersen Samplers Inc |
Low pressure impactor |
80-35000 (1) |
|
Hauke KG |
Berner impactor |
63-16700 (1) |
|
MSP corporation |
MOUDI |
56-18000 (1) |
|
MSP corporation |
Nano-MOUDI |
10 – 32 |
|
PIXE International Corp. |
Orifice impactor (model 1L-CI) |
60-16000 (1) |
|
Dekati |
Electrical low pressure impactor (instantaneous measurements) |
7- 10 000 |
F) Direct surface measurement devices
No instrument is fully adapted to personal or fixed station surface measurements for aerosols. The epiphaniometer is the only instrument developed for nanoparticles and specifically for this type of measurement. It can provide the total surface measurement of particles between 10 and 1000 nm. This instrument is bulky and has the disadvantage of using radioactive source, which complicates and restricts its use in the work environment. However, an aerosol’s specific surface can be measured from an aerosol’s granulometric distribution by assuming a specific geometry of the particles. Surface measurements can also be performed from samples collected by the Brunauer Emmett Teller (BET) adsorption method, using nitrogen or CO2. This technique allows measurement of all the inner surfaces of the pores. It thus gives a better estimate of the total surface presented by an aerosol than the measurement based on SEM or TEM electron microscopy. However, this method requires the use of large sample quantities and does not allow nanoparticles to be differentiated from other particles. The few commercially available devices are presented in Table 5.
|
Manufacturer |
Device |
Analytical range |
|
Micromeritics Instruments Co |
FlowSorb III 2305/2310 (B.E.T.) |
> 0.01 m²/g |
|
Paul Scherrer Institute |
Epiphaniometer4 |
0.003 μm² /cm³ |
|
Ecochem |
Standard Particulate Monitor LQ1-DC |
|
Table: 5 Particle surface determination devices
There is consensus on the current limitations of industrial hygiene measuring instruments and the need to develop new instruments adapted to nanoparticle specificities, as well as the absence of reference standard nanoparticles that can be used to calibrate instruments. Simultaneously, the availability of nanomaterials will allow development of new measuring tools applicable to a wide range of environmental situations, in the work environment, in the medical field, etc.
5. MARKETED PRODUCTS OF NANOTECHNOLOGY:
Nanotechnology today is growing very rapidly and has infinite applications in almost everything we do. The medicine we take, food we eat, chemicals we use, car we drive and much much more.
mknano offers large variety of nano products in various forms as mentioned below:
A) Carbon Nanotubes:
Single wall (SWNT), Double wall (DWNT), Multiwall (MWNT), (alligned/tangled/dispersable), OH, COOH Functionalized SWNT/MWNT, Industrial Grade SWCNTs, MWCNTs, Conducting (Metallic) and Semiconducting SWCNTs, MWCNT Nonwoven Papers, CNT Foam, Special application CNTs.
Other Nanotubes (Metals, Compounds, and Oxides/Hyroxides)
B) Quantum Dots:
Cadmium Mercury Telluride (CdHgTe), Cadmium Selenide (CdSe), Cadmium Selenide/Zinc Sulfide (CdSe/ZnS), Cadmium Sulfide (CdS), Cadmium Telluride (CdTe), Cadmium Telluride/Cadmium Sulfide (CdTe/CdS), Lead Selenide (PbSe), Lead Sulfide (PbS)
C) Nano Dry Lubricant Powders:
Tungsten Disulfide (WS2), Molybdenum Disulfide (MoS2), Hex-Boron Nitride (hBN), Graphite,
Specially formulated Nano Lubricant Additive Powders to improve lubricity and save energy.
D) Nano Powders:
Table: 6 list of nanopowders
|
Alumina Aluminum Aluminum nitride Aluminum oxide Antimony pentoxide Antimony tin oxide Brass Calcium carbonate Calcium chloride Calcium oxide Carbon black Cerium Cerium oxide Cobalt Cobalt oxide Copper Copper oxide Gold Hastelloy Hematite Indium Indium tin oxide |
Iron Iron-cobalt alloy Iron-nickel alloy Iron oxide Iron oxide, transparent Iron sulphide Lanthanum Lead sulphide Lithium manganese-oxide Lithium titanate Lithium vanadium- Magnesia Magnesium Magnesium oxide Magnetite Manganese oxide Molybdenum Molybdenum oxide |
Montmorillonite-clay Nickel Niobia Niobium Niobium oxide Silicon carbide Silicon dioxide Silicon nitride Silicon nitride- Silicon nitride- Silver Stainless steel Talc Tantalum Tin Tin oxide Titania Titanium |
Titanium diboride Titanium dioxide Tungsten Tungsten carbide-cobalt Tungsten oxide Vanadium oxide Yttria Yttrium Yttrium oxide Zinc Zinc oxide Zirconium Zirconium oxide Zirconium silicate |
E) Elements:
Ag; Al; Au; B; C (diamond); C (Graphite); Co; Cr; Cu; Fe; Mn; Mo Ni; Sn; Si; Ti; TiH2; W; Zn
F) Compounds:
AlN; B4C; BN (hexagonal/cubic); B3N4 (hex.); CaS; CrB; Cr3C2; CrN; FeS; GaN (spher.); GaP; HgI2; InP; LaB6; Mo2B; Mo2C; MoS2; NbC; NbN; PbS; SiC; Si3(C0.5N0.5)4; Si3N4; TaC; TaN; TiB; TiC; TiC0.8N0.2; TiC0.7N0.3; TiC0.5N0.5; TiN; VC; VN; WB; WC; WC/Co; WN; ZnS; ZrB2; ZrC; ZrN
G) Single Metal Oxides:
Al2O3; Al(OH)3; B2O3; Bi2O3; CeO2; CoO; Co3O4; CrO3; Cr2O3; CuO; Dy2O3; Er2O3; Eu2O3; Fe2O3; Fe3O4; Gd2O3; HfO2; In2O3; In(OH)3; La2O3; MgO; Mg(OH)2; Mn2O3; Mn3O4; MoO3; Nd2O3; NiO; Ni2O3; PbO; Pr6O11; Sb2O3; SiO2; Sm2O3; SnO2; Tb4O7; TiO2 (anatase/rutile); VO; V2O3; V2O5; WO3; Y2O3; ZnO; ZrO2
H) Multielement Oxides:
BaCO3; BaFe12O19; BaSO4; BaTiO3; CaCO3; Ca5(PO4)F; CoFe2O4; CuFe2O4; MgAl2O4; MgFe2O4; Li4Ti5O12; NiFe2O4; In2O3:SnO2; Li2CO3; LiCoO2; LiMn2O4; SrAl12O19; SrAl12O19; SrCO4; SrFe12O19; SrTiO3; Y3Al5O12 ZnFe2O4
I) Nanoparticle Dispersions:
Nanoparticle dispersions are available in water, 2-Propanol, Toluene, Ethylene Glycol etc.
J) Element Nanoparticle Dispersions:
Carbon (Nanodiamond), Carbon (Carbon nanotubes), Cobalt, Copper, Gold, Iron, Platinum, Silicon, Silver, Titanium
K) Oxide Nanoparticle Dispersions:
Aluminum Oxide (Al2O3), Iron Oxide (Red, Yellow), Silicon Oxide (SiO2), Titanium Dioxide (TiO2) Anatase/Rutile, Zinc Oxide (ZnO)
L) Rare Earth Oxide (REO) Nanoparticle Dispersions:
CeO2, Dy2O3, Er2O3, Gd2O3, Ho2O3, Sm2O3, Y2O3, ZrO2
6. Applications of nanotechnologies
The anticipated economic potential of nanotechnologies is phenomenal. It is predicted that a multitude of applications will be found for nanoparticles and affect many sectors of activity.
A) Automobile and aerospace industries:
reinforced and lighter materials; adhesives; more efficient electrical and magnetic rheological liquids; scratch-resistant, elastic, dirt-repelling exterior paints with colour effects; lighter, faster, safer vehicles; more durable and more reliable roads, bridges, pipelines and railway systems; anticorrosion coatings; sensors optimizing engine performance; ice detectors on aircraft wings, recyclable, longer-lasting tires; incombustible plastics.
B) Electronics and communications industries:
high-density memories and miniaturized processors; new solar cells, batteries and combustion cells; optoelectronic components, including lasers; faster processing speeds; greater data storage capacity; pocket electronic libraries; coupling silica with organic substances; logical digital components; ultra fast compact computers and electronic games with quantum electronics, wires and computers; brightly lit flat screens.
C) Chemical and materials industries:
multifunctional and more efficient ceramics, pigments, powders and catalysts; lighter and stronger wires; corrosion inhibitors and corrosion-resistant alloys; adhesiveless bonding technologies; new welding technologies; functional layers (thermal insulation, anti-adhesive, antistatic); photoactive and selfcleaning paints, windows and clothing; membranes for separation of materials (water treatment, dialysis); structured catalysts; ultra resistant coatings; extremely hard and resistant cutting tools.
D) Pharmaceutical, biomedical and biotechnology industries:
new drugs and active agents, including cosmetics, sun creams and protective creams; new antiallergenic medical adhesive surfaces; improvement of existing drugs, customized drugs delivered only to specific organs of the body; biocompatible surfaces for implants; nanoparticle-based oral vaccines; production of magnetic nanoparticles from biological media and production of biocompatible materials; tissue engineering and regeneration; neuron-transistor interfaces.
E) Healthcare:
Nanomaterials will allow physicians to do a better job of viewing, treating and repairing the body’s interior: the implanting of miniaturized diagnostic media to obtain early diagnoses; in surgery, tissue engineering and implants with nanotechnological coatings that can improve biocompatibility and bioactivity; multifunctional sensors; DNA analysis; manufacturing of ultraprecision devices, analytical and positioning systems, better optical systems; novel means of remedying severe handicaps such as deafness, blindness or certain paralyses; membranes for dialysis; preventive medicine with highly sensitive minisensors, microlaboratories, highdensity microchips; early diagnosis, prevention and treatment of cancer; biodetection of pathogens; protein detection, tissue engineering; destruction of tumours by heating.
F) Energy:
New generation of photovoltaic cells; more economical lighting; batteries and compact combustion cells with large internal surfaces; hydrogen storage in nanotubes; quantum dot lasers; more efficient conversion of solar and wind energy; intelligent windows; more efficient insulating materials.
G) Manufacturing sector:
precision engineering for production of new generations of microscopes and measuring instruments; new processes and new tools for manipulating matter at the atomic level; nanopowders incorporated into bulk materials with special properties, such as sensors that detect imminent failures and monitor measurement controls; manufacturing of biologically inspired materials.
H) Environment and ecology:
selective chemistry; colloidal membranes; selective catalysts; protection of sensitive organisms and reduction of CO2 emissions; functional non-toxic layers of multifunctional sensors for environmental depollution; production of ultrapure water from seawater, better use, recovery and recycling of existing resources, more efficient and less harmful pesticides and fertilizers; specific real-time chemical and multisubstance analyzers.
I) Process security:
compact zeolite reactors; new bonding technologies; production of copies (credit and debt cards, bank notes); adjustment of standards at the atomic scale, self-organized processes; quality control at the atomic scale; manufacturing processes with sensors leading to production with less defects.
J) Defense:
detectors of chemical and biological agents; much more efficient electronic circuits; much more resistant materials and coatings; light, high-performance textiles that repair themselves; miniaturized surveillance systems; more precise guidance systems.
K) Others uses:
- Some nanomaterials are already in commercial use. Examples include the use of metallic oxides in ceramics, zinc, iron, cerium and zirconium oxides, anti-scratch coatings for lenses, and in certain cosmetics and in sunscreens.
- It is estimated that the skin protection and skin care market uses 1000 to 2000 metric tons of metallic oxides annually.
- Clays with nanometric dimensions are integrated into certain materials to increase their strength, hardness, heat resistance and fire resistance.
- Nanotubes are already used as coating to minimize and dissipate static electricity in fuel lines and in electronics, in electrostatic paints and as flame retardants for certain plastics. Also, bandages, cardiac valves, non-streaking paints and unwrinklable and anti-stain fabrics containing nanometric components are being produced.
- Exploratory studies are under way to use quantum dots in diagnosis and medical therapy and for self-assembly of nanoelectric structures.
- Several composites displaying specific mechanical, optical, electrical or magnetic properties use nanoparticles. For example, monolayer or multilayer carbon fibers would allow control of the conductivity of certain plastics; they would be used in antistatic packaging. Carbon black, part of which has a nanometric dimension, is already widely used to reinforce tires. Composite materials based on nanometric-dimensioned clay and plastic are widely used, particularly for automobile bumpers.
- Although certain zeolites, titanium, zinc and iron oxides, carbon black and silica are the only nanoparticles currently produced in high tonnage, it nonetheless remains that, in the years ahead, nanoparticles will contribute to the improvement of a many products in various sectors. Major advances are expected in the short term in electronics, nanobiotechnology and nanomedicine.
7. NANOPARTICLES AND THEIR HEALTH EFFECTS
Nanoparticles have exceptional physical, chemical and electrical properties. What about their biological properties and interactions with the human body? Do they present a health risk for the workers who produce, handle, transform or use them?
There are two major reasons for modifying nanoparticle surfaces. First, a surface coating is frequently used to prevent aggregation of particles, but little data is available on the toxicity of these coated nanoparticles. Second, many modifications have been made to nanoparticle surfaces to modify their behavior in the human body and develop new medications.
several studies on the toxicity of specific nanoparticles (fullerenes, carbon nanotubes, quantum dots …) are available.

Figure: 20 Prediction of total and regional deposits of particles in the airway according to particle size (41). Reproduced with the authorization of INRS-France
Quantitative assessment of the risk of exposure to nanoparticles

Figure: 21 Effects of nanopareticles
The international experts assembled by the European Commission are unanimous that the potential deleterious effect of nanoparticles cannot be predicted from the toxicity of bulk materials of the same chemical composition but of greater size.
The risk assessment cannot be established precisely, since the dose-response relationships are insufficiently known. For most of the particles that can become airborne and breathed in, the primary concern is the potential damage to the respiratory system, which represents the most likely absorption route in the work environment.
The solubility of the particles directly affects their toxicity and the way they must be assessed or analyzed. In the presence of soluble particles, the entire mass deposited in the pulmonary air passages will quickly become available by dissolution in the biological fluids. This mechanism is well known for particles of larger dimensions. It is the same for nanoparticles. For soluble particles, the assessment of the total mass concentration of these particles thus will become a good indicator of their toxicity.
However, insoluble or low solubility particles will retain their form and expose their surface to the host organism. It then becomes important to document the specific toxicity of these insoluble or low solubility nanoparticles. In the absence of adequate knowledge, attention should also be paid to the cutaneous system and the possibility of ingestion of nanoparticles, particularly by adopting strictly hygienic safety measures.
To assess the potential human health effects of nanoparticles, it is important to develop knowledge that can provide answers to several questions. Specifically, it is essential to document how nanoparticles are absorbed into the human body by several routes (pulmonary, cutaneous and gastrointestinal), how nanoparticles are distributed in the body (blood, lymphatic system), the organs in which nanoparticles tend to accumulate significantly (lungs, brain, kidneys, liver…), and how nanoparticles are metabolized and then eliminated.
8. Future Opportunities and Challenges
Nanoparticles and nanoformulations have already been applied as drug delivery systems with great success; and nanoparticulate drug delivery systems have still greater potential for many applications, including anti tumour therapy, gene therapy, AIDS therapy, radiotherapy, in the delivery of proteins, antibiotics, virostatics, vaccines and as vesicles to pass the blood-brain barrier.
Nanoparticles provide massive advantages regarding drug targeting, delivery and release and, with their additional potential to combine diagnosis and therapy, emerge as one of the major tools in nanomedicine. The main goals are to improve their stability in the biological environment, to mediate the bio-distribution of active compounds, improve drug loading, targeting, transport, release, and interaction with biological barriers. The cytotoxicity of nanoparticles or their degradation products remains a major problem, and improvements in biocompatibility obviously are a main concern of future research.
There are many technological challenges to be met, in developing the following techniques:
a) -Nano-drug delivery systems that deliver large but highly localized quantities of drugs to specific areas to be released in controlled ways;
b) -Controllable release profiles, especially for sensitive drugs;
c) -Materials for nanoparticles that are biocompatible and biodegradable;
d) -Architectures / structures, such as biomimetic polymers, nanotubes;
e) -Technologies for self-assembly;
f) Functions (active drug targeting, on-command delivery, intelligent drug release devices/ bioresponsive triggered systems, self-regulated delivery systems, systems interacting with the body, smart delivery);
g) -Virus-like systems for intracellular delivery;
h) -Nanoparticles to improve devices such as implantable devices/nanochips for nanoparticle release, or multi reservoir drug delivery-chips;
i) -Nanoparticles for tissue engineering; e.g. for the delivery of cytokines to control cellular growth and differentiation, and stimulate regeneration; or for coating implants with --nanoparticles in biodegradable polymer layers for sustained release;
j) -Advanced polymeric carriers for the delivery of therapeutic peptide/proteins (biopharmaceutics),
And also in the development of:
k) -Combined therapy and medical imaging, for example, nanoparticles for diagnosis and manipulation during surgery (e.g. thermotherapy with magnetic particles);
l) -Universal formulation schemes that can be used as intravenous, intramuscular or peroral drugs
m) -Cell and gene targeting systems.
n) -User-friendly lab-on-a-chip devices for point-of-care and disease prevention and control at home.
o) -Devices for detecting changes in magnetic or physical properties after specific binding of ligands on paramagnetic nanoparticles that can correlate with the amount of ligand.
-Better disease markers in terms of sensitivity and specificity.
9. CONCLUSION
Nanotechnologies and nanoparticles represent a promising and fast-growing field. This is principally because a nanodimensional substance can have physical and chemical properties that are different from those of the same substance with larger dimensions. Indeed, current technological developments in this field are attempting to take advantage of these unique properties.
The number and diversity of exposed workers will increase over the next few years. It is possible to introduce prevention and control measures promoting occupational health and safety at the very beginning of the conception and implementation stages of various processes.
Consequently, these measures, if taken right away, can constitute an important asset for the field of nanotechnology. However, a major challenge remains since current knowledge concerning health risks in this field is very fragmentary.
Certain nanotechnology applications will entail few new occupational health and safety risks. One such field is electronics, where miniaturization is advancing rapidly and is now being measured nanometrically. By contrast, free nanoparticles in the air are cause for concern due to their potentially negative impact on occupational health and safety, their accumulation in the environment and their enrichment via the food chain. These could have long-term risks for the health of populations. Although current knowledge on the toxicity of nanoparticles and the potential level of worker exposure is very limited, preliminary results in most of the important studies reveal significant biological activity and adverse effects.
In the short term, it will be almost impossible to acquire adequate knowledge of the risk associated with every type of nanoparticle. This is due not only to the proliferation of new nanoparticles but also to the modifications made to their surfaces; for these modifications greatly affect the surface properties of nanoparticles and possibly their biological reactivity and toxicity as well.
Given that it is currently impossible to carry out precise, quantitative risk assessment for every type of particle, it is important to develop a precautionary approach, using strategies based on prevention, sound practices and risk control that can inhibit the spread of occupational disease.
In the area of industrial hygiene, initiatives must be taken as soon as possible, using the best available measuring tools -- in spite of these tool’s limitations -- to estimate levels of occupational exposure. In view of the rapid advances in knowledge in occupational health and safety, it would seem important to update our assessment of knowledge in this field as soon as possible.
Thus nanoparticles provides many diseased as well as much more applications in world.
10. BIBLIOGRAPHY
1. Khar Roop K. and Jain N.K. : Solid lipid nanoparticle as Novel Nanoparticle system in Targeted and controlled drug delivery.
2. Jain N.K. : Novel drug delivery system in book of Advance in controlled and novel drug delivery system .
3. Ujela shely and Jain n.k. : Solid lipid nanoparticle .
4. Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci [serial online] 2009 [cited 2010 Apr 22];71:349-58.
5. Jumaa M, Muller BW. Lipid emulsions as a novel system to reduce the hemolytic activity of lytic agents: Mechanism of protective effect. Eur J Pharm Sci 2000;9:285-90.
6. Cavalli R, Caputo O, Gasco MR. Solid lipospheres of doxorubicin and idarubicin. Int J Pharm 1993;89:R9-R12.
7. Gasco MR. Method for producing solid lipid microspheres having a narrow size distribution. United states patent, US 188837; 1993.
8. Muller RH, Runge SA. Solid lipid nanoparticles (SLN) for controlled drug delivery. In: Benita S, editor. Submicron emulsions in drug targeting and delivery, Amsterdam: Harwood Academic Publishers; 1998. p. 219-34.
9. Jenning V, Gysler A, Schafer-Korting M, Gohla S. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm 2000;49:211-8.
10. Muller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drug. Int J Pharm 2002;242:121-8.
11. Radtke M, Muller RH. Comparison of structural properties of solid lipid nanoparticles (SLN) versus other lipid particles. Proc Int Symp Control Rel Bioact Mater 2000;27:309-10.
12. Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie 2006;61:375-86. [PUBMED] [FULLTEXT]
13. Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 2002;54:S131-55.
14. Souto EB, Wissing SA, Barbosa CM, Muller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm 2004;278:71-7.
15. Souto EB, Muller RH. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J Microencapsul 2006;23:377-88.
16. Souto EB, Muller RH. SLN and NLC for topical delivery of ketoconazole. J Microencapsul 2005;22:501-10.
17. Souto EB, Muller RH. The use of SLN and NLC as topical particulate carriers for imidazole antifungal agents. Pharmazie 2006;61:431-7.