ABOUT AUTHORS:
S.M Abdullah1*, M Tandon1, N Saha2, K K Pillai3
1M.Pharm, Department of Clinical Pharmacology Unit, Ranbaxy Laboratories Ltd, India.
2Department of Medical Affairs & Clinical Research, Ranbaxy Laboratories Ltd, India.
3Ph.D, Department of Pharmacology, Faculty of Pharmacy, Jamia Hamdard, India.
SUMMARY
The principle for the treatment of hypertension as per JNC-7 guideline is treat to blood pressure (BP) <140/90 mm Hg but in common clinical practice it is often difficult to lower the BP below 140/90 mm Hg with monotherapy. While majority of patients requires two or more drugs to achieve target BP, this finally calls for comparative clinical evaluation of antihypertensive drugs as co-administration verses monotherapy.
METHOD: This study was randomized, single-blind, double-dummy, three-treatment, crossover study included newly diagnosed treatment naive essential hypertensive patients; aged 20-45 years with sitting office BP ranged 140-159/94-104 mmHg. After 4 weeks of regular measurements of BP, all subjects received each treatment in randomized order for 1 week: imidapril 5 mg and placebo (I/P), lercanidipine 10 mg and placebo (L/P) or imidapril 5 mg and lercanidipine 10 mg (I/L). Prior to dosing and at the end of each treatment period, 24-hours ambulatory blood pressure monitoring (ABPM) was performed. Recorded data was analyzed for the evaluation of pharmacodynamic potential of each treatment.Beside this time-effect profile was also evaluated in terms of trough-to-peak (T/P) ratio.
RESULTS: Normalization rate and antihypertensive potential were found to be higher with I/L treatments than I/P and L/P alone.Mean change in BP were reported 9.23/6.5, 4.35/3.28 and 6.76/4 with I/L, I/P and L/P respectively.In each treatment group, T/P ratio was greater than 0.5 except in co-administration treatment group DBP was lesser than 0.5. All reported adverse event were primarily of mild intensity. P-value ≤ 0.05 was considered significant. Values are represented as mean±SD.
CONCLUSION: The co-administration may have clinically synergistic effects with less adverse events than monotherapy. Further, additional clinical studies on larger pool of hypertensive subjects are warranted.
Reference ID: PHARMATUTOR-ART-1960
INTRODUCTION
Hypertension is a condition that afflicts almost 1 billion people worldwide and is a leading cause of morbidity and mortality. It is estimated that hypertension contribute to approximately 5% of the global disease burden(Whitworth 2003). World Health Organization in 1999 reports that one out of every three deaths in India is due to heart diseases.
Combination therapy may be theoretically favored by the fact that multiple factors contribute to hypertension, and achieving control of blood pressure with single agent acting through one particular mechanism may not be possible. Regimens can either be fixed dose combinations or drugs added sequentially one after other. Combining the drugs makes them available in a convenient dosing format, lower the dose of individual component, thus, reducing the side effects and improving compliance. Classes of antihypertensive agents which have been commonly used are angiotensin receptor blockers, thiazide diuretics, beta and alpha blockers, calcium antagonists and angiotensin-converting enzyme inhibitors. Thiazide diuretics and calcium channel blockers are effective, as well as combinations that include renin-angiotensin-aldosterone system blockers, in reducing BP. Combinations may be individualized according to the presence of comorbidities like diabetes mellitus, chronic renal failure, heart failure, thyroid disorders and for special population groups like elderly and pregnant females. All antihypertensive medications can be combined as necessary but some combinations make more sense than others. Proper understanding of the underlying mechanism by which various classes of antihypertensive drugs act together, provides the rationale and logical approach for developing effective combination (Naidu et al. 2000). One of the allegedly rational combinations includes, ACE-inhibitor(ACEI) plus calcium channel blocker (CCB) (Fogari et al. 2006; Rump et al.2006; Ragot et al.2006).
CCBs are potent vasodilators; induces reflex activation of the sympathetic nervous system and renin angiotensin system. Besides this CCBs create an imbalance of hydrostatic force in peripheral capillaries, and facilitates fluid extravasations into the interstitial space, which ultimately causes the formation of lower extremity edema due to the gravitational force(Kohlmann 2006). Combination with ACEI leads to buffering of CCB induced endocrine activation. Hence, combination of CCB with ACEI is theoretically sound(Naidu et al. 2000). Furthermore, it is necessary to call for a comparative pharmacodynamic study between ACEI (imidapril 5 mg) and CCB (lercanidipine 10 mg)as monotherapy and their co-administration in essential hypertensive patients. Imidapril and lercanidipine are being used for the treatment of mild to moderate essential hypertension.
Since, ambulatory blood pressure monitor have several potential advantages over the sphygmomanometer, like no observer bias or digit preference, greater reproducibility, little or no white-coat hypertension effect and assessment can be done under the conditions of daily life. Therefore, ABPM was chosen as blood pressure recording device for this study.
MATERIALS AND METHODS
The protocol and the corresponding Informed Consent Form used for this study were approved by the Fortis Hospital Institutional Ethics Committee (FHIEC). The study was conducted at the Ranbaxy Clinical Pharmacological Unit (CPU), Noida, India.
A total of fifteen, newly diagnosed treatment naive essential hypertensive male subjects were enrolled in a randomized, single-blind, double-dummy, three-treatment, three-period, crossover study (figure-1). The treatment sequence was balanced using Latin Square design. Subjects, in the age range from 20 to 45 years were selected from the volunteer bank of CPU Noida, India.
The regular measurement of blood pressure was carried out at a weekly interval for one month prior to the screening of the subjects for the study. During the run-in period, they were advised to take balanced diet (salt intake <6 gm/day and low fat meals with moderate calories), to do regular physical exercises and to weekly report themselves at CPU. At the end of one month, subjects with an office sitting systolic blood pressure {SSBP} in range of 140-159 mmHg (inclusive), and office sitting diastolic blood pressure {SDBP} in range of 94-104 mmHg were selected for screening assessments.
One week prior to admission of this study, a complete physical examination and laboratory screening was performed; laboratory screening consisted of haematology examination, blood chemistry including serum creatinine, serum potassium, serum sodium, alkaline phosphatase, and amino transferase, and TSH level. Subjects with history or evidence of significant target organ damage, heart or cerebrovascular disorder, severe renal and hepatic impairment, a history of alcoholism, or other drug abuse, abnormal thyroid function test, mental impairment, and any medical or surgical conditions or medications known to interfere with the absorption and metabolism of the study drugs were excluded.All subjects were instructed to abstain from smoking and exercise in the duration of the study. Subjects were admitted after obtaining consent prior to the study and they were in-housed approximately for 9 days in each period. ABPM device (Space Labs Model 90207) was applied for 24 hours prior to 1st dosing and after 7th dosing of the study medications in each period.
All the pharmacodynamic parameters were assessed on the basis of ‘hourly average tabular data’ generated by ABPM device. To measure the time-effect profile in terms of trough-to-peak (T/P) ratio the peak effect was assessed by concentrating on the interval from 2 to 8 hours after dose administration, with the largest mean difference from baseline over a 2-hours period being used for the estimate. The trough blood pressure for each treatment is the difference from baseline between 22 to 24-hours after dose administration (Omboni S et al. 2005). The T/P ratio is calculated from the mean peak and trough values of each treatment group.
After 24-hours baseline recording, dosing was done under overnight fasting conditionat 9:00 AM on next morning. Subjects were continued under fasting condition for another 0.5 hours after the administration of dose. This was followed by a standardized lunch, snacks and dinner at 5, 9 and 13 hours post drug administration respectively.
In this study, two different class of antihypertensive drugs were administered- [Tanatril® (ACEI) containing 5 mg imidapril hydrochloride, Elder Pharmaceuticals Limited, India, Batch # 700102 and Lerka® (CCB) containing 10 mg lercanidipine-hydrochloride, Sun Pharmaceutical Limited, India, Batch # SK71552]. The subjects were randomized in such a way that they received each of the study treatments: lercanidipine 10 mg plus placebo (L+P) as monotherapy or imidapril 5 mg plus placebo (I+P) as monotherapy or co-administration of lercanidipine 10 mg plus imidapril 5 mg (L+I). All study medications were placed individually in a hard gelatine capsule shell of same colour and dimension to comply with blinding. Each study medications were administered as single oral daily dose for seven days. All treatments were received by each subject with a washout period of six days.For, clinical safety purpose, brief clinical examination and vitals were conducted at intervals of 12 and 4-hours respectively; 12-Lead electrocardiogram was recorded at 6 hours post dose in each period.At the end of study, clinical laboratory tests including hematology and biochemistry were also performed.
Data summarization and statistical analyses were performed using the Graph Pad Software System (Graph Pad Software System Inc, 11452 EI Camino Real, # 215, San Digeo 92130, USA) in which one way ANOVA followed by Tukey tests were applied to compare the potential pharmacodynamic effects of co-administration and monotherapy. Differences were considered significant for P values, equal or less than 0.05. All values are reported as mean ± standard deviation (SD).
RESULTS
The study was conducted in an Asian male population. The average age (year), weight (kg) and height (cm) of the subjects were measured 31.00±9.10, 60.06±7.95 and 165.93±5.92 respectively. This study was completed over a period of five weeks. All enrolled subjects completed the study except two of them who were dropped out (one prior to drug administration and another after completion in 1st period, because of their personal reasons).
Blood pressure lowering potential of imidapril (5 mg) and lercanidipine (10 mg) as monotherapy and their co-administration was compared with the respective baseline. In each treatment group, mean 24 hours, daytime, nighttime SBP and DBP were significantly lowered except that of nighttime DBP. The lowering in nighttime SBP was also found not significant in subjects who were received 5 mg imidapril tablets as monotherapy. After last dosing of imidapril, lercanidipine and co-administration, the mean difference in ABPM 24 hours SBP and DBP were 4.35/3.28, 6.76/4.00 and 9.23/6.50 mmHg respectively from the respective baseline value (Figure-2). Continuous change in ABPM 24 hr SBP and DBP profile against the baseline (figure-3).Normalization rates (<90 mmHg) were observed at trough was much better in co-administration therapy 75.62% than the monotherapy of lercanidipine 60% and imidapril 58.33%(figure-4).
Co-administration therapy was significantly superior to each monotherapy in reducing ambulatory (24hr/daytime/night-time) SBP and DBP, while monotherapy seems to be adequate to lower the blood pressure with statistically significant magnitude only in daytime rather than in night-time(Table1).
The T/P ratios of the treatment groups were calculated by dividing the average change in trough systolic and diastolic pressure by the average change in peak systolic and diastolic pressure. All subjects were included in this analysis. In each treatment group the T/P ratio was greater than 0.5 except in co-administration treatment group, where DBP was found to be lesser than 0.5 (Table 2).According to US FDA proposed guidelines for the clinical evaluation of new antihypertensive drugs, suggesting that the drug effect at trough should be not less than half to two thirds of the peak (US DHHS, 1988). In this clinical study, results showed that co-administration reduces the ambulatory blood pressure more potentially than monotherapy in daytime. But the monotherapy produced a homogenous ambulatory blood pressure control over the 24-hours than co-administration therapy. As T/P ratio was observed greater with respect to monotherapy in comparison to co-administration therapy.
Lercanidipine 10 mg and imidapril 5 mg were well tolerated when administered either alone or co-administered. Calamine lotion was given to a subject who was on co-administration therapy and developed rashes on his back which was further subsidized after its application. Except that, only few cases of study drugs related mild adverse events were observed which were further resolved without anysequelae or need for drug treatment. All adverse events intended to study medications are summarized in table-2.
BP measurement with ABPM device had several potential advantages over sphygmomanometer BP measurement, which includes - No observer bias or digit preference, greater reproducibility, little or no white-coat hypertension effects, easy to differentiate dipper and non-dipper hypertensive subjects and assessment under conditions of daily life for full 24 hour period.
CONCLUSION
In conclusion, the results of present study showed that all study treatments were effective in lowering ambulatory blood pressure in subjects with the essential hypertension, but co-administration therapy was significantly more effective than monotherapy.
However, the treatment with higher trough/peak ratio (monotherapy) produces a much more consistent effect over 24 hours. In comparison, co-administration produces a profound fall in blood pressure around the time of peak response which then tails off towards the trough effect at the end of the dose. Furthermore, a direct comparison of monotherapy and co-administration demonstrated superiority for monotherapy with respect to trough/peak ratio.
In respect to monotherapy, comparatively less adverse events were occurred in a group who received co-administration therapy. Therefore, it shows that the improved efficacy with co-administration was not associated with a worse tolerability of the treatment.
Finally, it concludes that lercanidipine 10 mg and imidapril 5 mg may be an effective combination for the treatment of essential hypertensive subjects. Further, additional clinical studies for the evaluation of this combination are warranted on larger pool of hypertensive subjects.
Figure-1
Study design
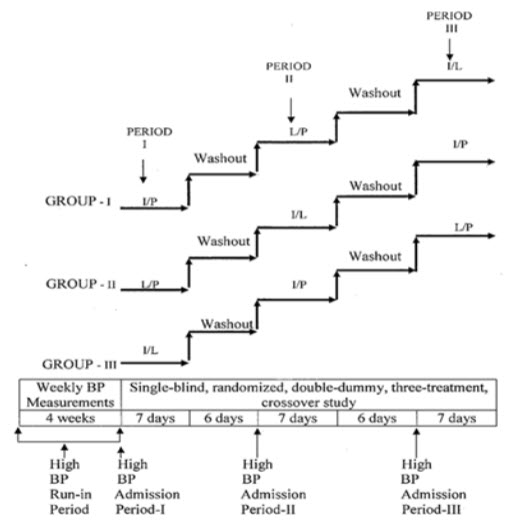
Where, Treatment I/P = Imidapril (5 mg) + Placebo (10 mg); Treatment L/P = Lercanidipine (10 mg) + Placebo (5 mg); Treatment I/L = Imidapril (5 mg) + Lercanidipine (10 mg).
Figure-2
Mean change in ABPM systolic and diastolic blood pressure from baseline after one week of treatments
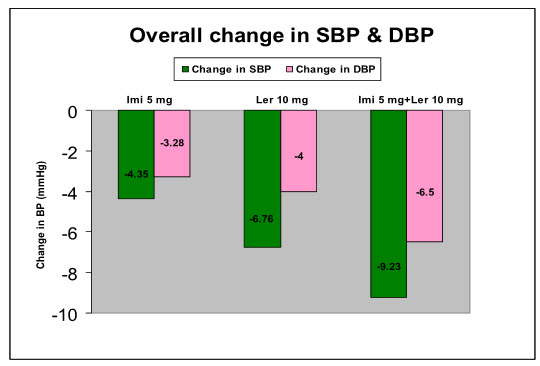
[Where, Imi = Imidapril (5 mg); Ler = Lercanidipine (10 mg)]
Figure-3
Systolic (upper panel) and diastolic (lower panel) blood pressure profile during baseline (B) and post treatment with Lercanidipine (L), Imidapril (I) and Co-administration (I/L)
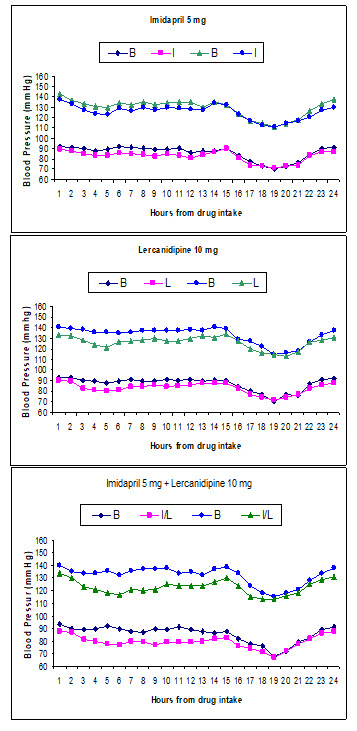
Figure-4
Normalization rates according to ABPM reading at trough
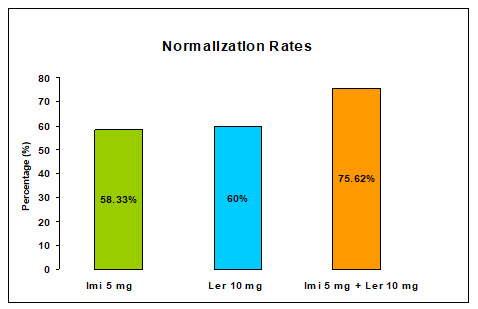
|
Pharmacodynamic Parameters |
TREATMENT-A |
TREATMENT-B |
TREATMENT-C |
|||
|
Baseline (N=14) |
Imi 5mg + Placebo (N=14) |
Baseline (N=13) |
Ler 10mg + Placebo (N=13) |
Baseline (N=13) |
Ler 10mg+ Imi 5mg (N=13) |
|
|
ABPM 24 h SBP |
129±9 |
125±7** |
133±8 |
126±6** |
132±7 |
123±6** |
|
ABPM 24 h DBP |
86±7 |
82±5** |
87±7 |
83±5** |
86±7 |
79±5** |
|
Daytime SBP |
134±3 |
129±4** |
137±2 |
129±3** |
136±2 |
125±5** |
|
Daytime DBP |
90±2 |
85±3** |
90±2 |
85±3** |
89±2 |
81±4** |
|
Night-time SBP |
118±5 |
116±4^ |
122±6 |
119±5* |
123±7 |
118±5* |
|
Night-time DBP |
77±5 |
76±5^ |
78±6 |
77±4^ |
77±5 |
74±5* |
Table-1
Ambulatory BP during baseline and post treatment with imidapril 5 mg, lercanidipine 10 mg and their co-administration for period of one week (Mean±SD)
** Highly Significant (P<0.01); * Significant (P<0.05); ^ Not Significant (P>0.05) as compared to respective baseline value.
Table-2
Trough/Peak ratio index in the three treatment groups after 1 week of treatment
|
SL No. |
Index |
Imidapril |
lercanidipine |
Imidapril + lercanidipine |
|
1. |
SBP Trough/Peak Ratio |
0.75 |
0.54 |
0.52 |
|
2. |
DBP Trough/Peak Ratio |
0.58 |
0.56 |
0.38 |
REFERENCE
1. Puig, J. G., Calvo, C., Luurila, O., Luurila, H., Sulosaari, S., and Strandberg, A. (2007) ‘Lercanidipine, enalapril and their combination in the treatment of elderly hypertensive patients: placebo controlled, randomised, crossover study with four ABPM’ Journal of Human Hypertension 21, 917-24
2. Miller-Craig, M., Shaffu, B., Greenough, A., Mitchell, L., and McDonald, C. (2003) ‘Lercanidipine vs lacidipine in isolated systolic hypertension’ Journal of Human Hypertension 17, 799-806
3. Ding, P, Y-A., Chu, K-M., Chiang, H-T., Shu, K-H. (2004) ‘A double-blind ambulatory blood pressure monitoring study of the efficacy and tolerability of once-daily telmisartan 40 mg in comparison with losartan 50 mg in the treatment of mild-to-moderate hyoertension in Taiwanese patients’ Int J Clin Pract 58, (Suppl. 145) 16-22
4. Naidu, M. U. R., Usha, P. R., Rao, T. R. K., and Shobha, J. C. (2000) ‘Evaluation of amlodipine, lisinopril, and a combination in the treatment of essential hypertension’ Postgrad Med J 76, 350-53
5. Kohlmann, O. Jr. (2006) ‘The “LOTHAR” Study: Evaluation of efficacy and Tolerability of the Fixed Combination of Amlodipine and Losartan in the Treatment of Essential Hypertension’ Arquivos Brasileiros de Cardiologia 86, N0 1
6. Fogari, R., Mugellini, A., Zoppi, A., Lazzari, P., Destro, M., and Rinaldi, A. (2006) ‘Effect of telmisartan/ hydrochlorothiazide vs. lisinopril/ hydrochlorothiazide combination on ambulatory blood pressure and cognitive function in elderly hypertensive patients’ J Hum Hypertens 20, (3) 177-85
7. Ragot, S., Sosner, P., Yau, C., Brunel, P., and Herpin, D. (2006) ‘Management in general practice of hypertensive patients poorly controlled with a fixed-dose renin–angiotensin system inhibitor and diuretic combination: results from a French national survey’ J Hum Hypertens 20, (6) 407-18
8. Rump, L. C., Ambrosioni, E., Burnier, M., Horl, W., and Rabelink, A. J. (2006) ‘Initial combination therapy with olmesartan/hydrochlorothiazide in moderate-to-severe hypertension’ J Hum Hypertens 20, (4) 299-301
9. Whitworth, J. A. (2003) ‘World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension’ J Hypertens 21, (11) 1983-92
10. Omboni S., Parati G., Zanchetti A., Mancia G. (1995) ‘Calculation of trough:peak ratio of antihypertensive treatment from ambulatory blood pressure. Methodological aspects. J Hypertens 13:1105-1112
11. Food and Drug Administration, Proposed Guideline for the Clinical Evaluation of Anti-Hypertensive Drugs. Rochville, MD: Division of Cardio-Renal Drug Products, US Department of Health and Human Services, 1988
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









