{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Paras Virani1,2*, Parul Jain3, Hasumati Raj2, Vineet Jain2
1Research Scholar 2014, Gujarat Technological University, Gujarat
2Quality assurance department, Shree Dhanvantary Pharmacy College, Kim, Surat
3Quality assurance department, Maliba Pharmacy College, Bardoli, Surat
parasvirani@gmail.com
ABSTRACT
Analytical method is primary requirement of the pharmaceutical industry. In pharmaceutical industry various analytical methods is used like chromatography, spectroscopy method, electrochemical method, ion incorporating method, etc. Irbesartan is classified as an angiotensin II receptor type 1 antagonist. Angiotensin II receptor type 1 antagonists are widely used in treatment of diseases like hypertension, heart failure, myocardial infarction and diabetic nephropathy. Atorvastatin is the most efficacious of the currently available HMG-CoA Reductase inhibitors used in anti lipidemic and also used in atherosclerosis, stroke and cardiac risk. In recently approved new fixed dose combination of Irbesartan and atorvastatin in market of Korea so it require analytical method development which help in industry for new drug delivery system development. This review highlights the role, need and application of various analytical techniques for Irbesartan and atorvastatin combination and their corresponding analytical methods in the pharmaceutical industry.
INTRODUCTION:
In the field of pharmaceutical research, the analytical investigation of bulk drug materials, intermediates, drug products, drug formulations, impurities and degradation products and biological samples containing the drugs and their metabolites is very important. From the commencement of official pharmaceutical analysis, analytical assay methods were included in the compendial monographs with the aim to characterize the quality of bulk drug materials by setting limits of their active ingredient content. In recent years, the assay methods in the monographs include titrimetry, spectrometry, chromatography and capillary electrophoresis; also the electro analytical methods can be seen in the literature.[1]
Hypertension frequently coexists with hyperlipidaemia and both are considered to be major risk factors for developing cardiac disease ultimately resulting in adverse cardiac events. This clustering of risk factors is potentially due to a common mechanism. Further, patient compliance with the management of hypertension is generally better than patient compliance with hyperlipidaemia. It would therefore be advantageous for patients to have a single therapy which treats both of these conditions.[2]
Coronary heart disease is a multifactorial disease in which the incidence and severity are affected by the lipid profile, the presence of diabetes and the sex of the subject. Incidence is also affected by smoking and left ventricular hypertrophy which is secondary to hypertension. To meaningfully reduce the risk of coronary heart disease, it is important to manage the entire risk spectrum. For example, hypertension intervention trials have failed to demonstrate full normalization in cardiovascular mortality due to coronary heart disease. Treatment with cholesterol synthesis inhibitors in patients with and without coronary artery disease reduces the risk of cardiovascular morbidity and mortality and beneficial effect for treatment in coronary heart disease.[3]
Irbesartan, an angiotensin II receptor antagonist, is used mainly for the treatment of hypertension. It is an orally active nonpeptide tetrazole derivative. IUPAN name of irbesartan is 2-butyl-3-({4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1,3-diazaspiro[4.4]non-1-en-4- one. These are organic compounds containing a biphenyl attached to a tetrazole. Atorvastatin is a member of the drug class known as statins. It’s mainly used in antilipidemic agent in cardiac risk condition. It is used for lowering cholesterol. Atorvastatin is a competitive inhibitor of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-determining enzyme in cholesterol biosynthesis via the mevalonate pathway.[4, 5]
In recently Hanmi pharmaceutical give patent for the combination use of newly develop combination of antihypertensive and antilipidemic drug for coronary artery disease.[6] here the antihypertensive agent used as irbesartan and antilipidemic agent is atorvastatin give safely and effective treatment. it is mainly used in to the hypertension with diabetic patient and also for cholesterol lowering purpose. This combination of atorvastatin and antihypertensive agents like irbesartan and losartan treat subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidaemia and to treat subjects presenting with symptoms of cardiac risk, including humans. additive and synergistic combinations of atorvastatin and irbesartan whereby those synergistic combinations are useful in treating subjects suffering from angina pectoris, atherosclerosis, combined hypertension and hyperlipidaemia and those subjects presenting with symptoms of cardiac risk, and congestive heart failure and coronary artery disease.
Irbesartan and atorvastatin Combination is also used in Postprandial Endothelial Dysfunction, Oxidative Stress, and Inflammation in Type 2 Diabetic Patients.[7] The possibility of reducing NT generation during acute hyperglycaemia with irbesartan. Atorvastatin and angiotensin and type 1 (AT-1) receptor blockers (irbesartan) are widely used in preventing CVD and diabetic complications, and it has been suggested that many of their ancillary effects are due to strong intracellular antioxidant activity. Mechanisms underlying the biological effects of atorvastatin and irbesartan differ, even in terms of intracellular antioxidant activity.
Recently no formulation available in USA, INDIA and other country but the fixed dose combination is taken marketing approval in Korea for combination of Irbesartan and atorvastatin. So this new combination requires analytical method development for helping to Pharma industry for new formulation development, clinical tails, and preformulation study.
ANALYTICAL METHOD DEVELOPMENT:
From the stages of drug development to marketing and post marketing, analytical techniques play a great role, be it understanding the physical and chemical stability of the drug, impact on the selection and design of the dosage form, assessing the stability of the drug molecules, quantitation of the impurities and identification of those impurities which are above the established threshold essential to evaluate the toxicity profiles of these impurities to distinguish these from that of the API, when applicable and assessing the content of drug in the marketed products. The analysis of drug (Irbesartan and atorvastatin) and its metabolite which may be either quantitative or qualitative is extensively applied in the pharmacokinetic studies. This review highlights the role of various analytical techniques and their corresponding analytical methods in the analysis of pharmaceuticals.[8]
The present state-of-the art for analytical method is replicated through the data in Table based on the edition of European (The European Pharmacopoeia and Council of Europe, 2002) and US (United States Pharmacopoeia, 2004) pharmacopoeias.
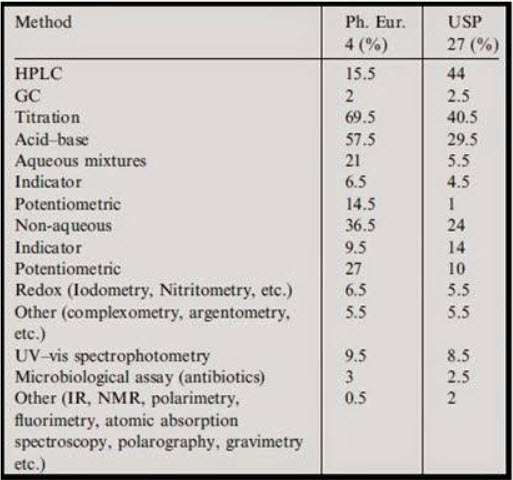
Figure 1: analytical method according EP and USP[9]
NEED OF ANALYTICAL METHOD DEVELOPMENT FOR IRBESARTAN AND ATORVASTATIN IN INDUSTRY
Following application of analytical method development for new fixed dose combination of Irbesartan and atorvastatin in pharmaceuticals:
- New drug delivery system :- Development of new dosage form like solid oral dosage form, parentral dosage form, modified release or sustained release form, etc.
- Clinical trial: - Analytical method is help for phase I and ll study on the patent.
- Preformulation study: - here the method helps to check API and excipient stability, excipient excipient stability, formulation testing, etc.
- Impurity profiling: - analytical method helps to finding out the impurity available in the formulation in the time of development. Also to checked the related substance present in the active pharmaceutical ingredient.
- Stability study: - high performance liquid chromatography helps to checked the stability profile of the drug substance in combination of Irbesartan and atorvastatin.
- Compatibility study:-irbesartan and atorvastatin combination compatibility study done using UV spectroscopy method, high performance liquid chromatography, high performance thin layer chromatography, etc.
- Identification study: - identification of drug or its combination by using the molecular analytical methods like infrared spectroscopy, nuclear magnetic resonance, spectroscopy, etc.
- Dosage form development
- Assay method
- Pharmacopoeial testing.
ANALYTICAL METHOD FOR IRBESARTAN AND ATORVASTATIN:
Following analytical method is used for the combination of two drugs in the pharmaceutical industry –
- Titrimetric techniques
- Chromatographic techniques
- Thin layer chromatography
- High performance thin layer chromatography
- High-performance liquid chromatography
- Gas chromatography
- Spectroscopic techniques
- Spectrophotometry
- Derivative spectroscopy
- Visible spectrophotometry
- Near infrared spectroscopy
- Fluorimetry and phosphorimetry
- Electrochemical methods
- Nuclear magnetic resonance spectroscopy
- Electrophoretic methods
- Kinetic method of analysis
- Flow injection and sequential injection analysis
- Hyphenated techniques.
From the literature survey of single Irbesartan and single atorvastatin and its combination with other drug in various analytical method available like Titrimetric techniques, chromatographic techniques, thin layer chromatography, high performance thin layer chromatography, high-performance liquid chromatography, spectroscopy, electrophoretic methods, etc.
|
Method |
Irbesartan |
Atorvastatin |
|
Irbesartan + Atorvastatin |
||
|
HPLC |
√ |
√ |
- |
|
||
|
RPHPLC |
√ |
√ |
- |
|
||
|
HPTLC |
- |
√ |
- |
|
||
|
UV SPECTROPHOTOMETRY |
√ |
√ |
- |
|
||
|
RP-UPLC |
√ |
√ |
- |
|
||
|
Stability indicating by HPLC(17 |
√ |
√ |
- |
|
||
|
LC MS/MS |
√ |
√ |
- |
|
||
|
Miscellaneous (includes Voltammetry, Spectrofluorometry, Capillary Electrophlorosis) |
√ |
√ |
- |
|
||
Table 1: literature summery of Irbesartan and Atorvastatin[FROM LITERATURE]
CONCLUSION:
From the literature survey no method available for fix dose combination of Irbesartan and atorvastatin. Recently Hanmi pharmaceutical take patent on bilayer tablet and solid oral dosage form of Irbesartan and atorvastatin so the formulation of this two drug is require for single therapy of hypertension and hyperlipidaemia. The development of new dosage form in pharmaceutical industry requires analytical method for estimation, assay, impurity, stability, etc. In development of new dosage form for Irbesartan and atorvastatin primary requirement is development of new analytical method and it’s apply for various purpose of identification, impurity profiling, assay estimation and other application.
REFERANCES
1. J. Ermer and McB. Miller. Method validation in pharmaceutical analysis. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, 20 – 25.
2. Virani P, Sojitra R, Raj H and Jain V; A review on Irbesartan co administered with Atorvastatin for the treatment of cardiac risk; Journal of Critical Review; 2014; 1(1); 25-28.
3. Antonio ceriello, Roberta assaloni, Roberto da ros, amabile maier, ludovica piconi, Lisa quagliaro, Katherine esposito and Dario giugliano. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation- American heart association. 2013; 111; 2517- 2524.
4. Virani P, Sojitra R, Raj H and Jain V; Irbesartan: A review on analytical method and its determination in pharmaceuticals and biological matrix; Inventi Rapid: Pharm Analysis & Quality Assurance; 2014; 4; 1-6.
5. Virani P, Sojitra R, Raj H and Jain V; Atorvastatin: A review on analytical method and its determination in pharmaceuticals and biological matrix.” Inventi Rapid: Pharm Analysis & Quality Assurance; 2014; 4; 1-6.
6. Hanmi pharmaceutical company limited; Efficacy and safety of Irbesartan and Atorvastatin in hypertension and hyperlipidaemia; March 2013;www.clinicaltrials.gov/show/NCT01442987; 2013
7. D. Allan butterfielda, B. Eugenio baronec, cesare mancusoc; Cholesterol- independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacological research; 2011; 64; 180-186.
8. Charles G. Smith; The Process of New Drug Discovery and Development. Informa Healthcare USA, Inc, 2006; 2(1); 36 – 40.
9. Gorog subrosh; regulatory requirement for analytical method; Journal of Pharmaceutical and Biomedical Analysis; 2005; 931–937.
REFERENCE ID: PHARMATUTOR-ART-2313
|
PharmaTutor (ISSN: 2347 - 7881) Volume 3, Issue 2 Received On: 08/12/2014; Accepted On: 12/12/2014; Published On: 01/02/2015How to cite this article: P Virani, P Jain, H Raj, V Jain; Need and Application of Analytical Method Development on New Fixed Dose Combination of Irbesartan and Atorvastatin in Pharmaceutical Industry; PharmaTutor; 2015; 3(2); 43-47 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









