About Authors:
Krunal Parikh1*, Mr. Maheshkumar Kataria2, Jatin Patel1
2Assistant professor, Department of pharmaceutics,
1Seth G.L. Bihani S.D. College of Technical Education,
Institute of Pharmaceutical Sciences and Drug Research,
Sri Ganganagar, Rajasthan, INDIA
*Krunal_2922@yahoo.in
ABSTRACT
Documentation is an integral part of good manufacturing practices. It defines a system of information and control so that risks so inherent in misinterpretation and/or error in oral communication are minimized. It consequently strengthens the quality, and its consistency, of all goods and services, as those responsible for the specific operations have clear, unambiguous instructions to follow including active drug substances, is legally mandatory.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1525
INTRODUCTION
PRINCIPLE
Documentation is an integral part of good manufacturing practices. It defines a system of information and control so that risks so inherent in misinterpretation and/or error in oral communication are minimized.
It consequently strengthens the quality, and its consistency, of all goods and services, as those responsible for the specific operations have clear, unambiguous instructions to follow including active drug substances, is legally mandatory. (OPPI Guideline)
OBJECTIVES OF DOCUMENTS
1. To define the specifications and procedures for all materials and method of manufactured and control.
2. To ensure that all personal concern with manufacture know what to do and when to do it.
3. To ensure that authorized persons have all the information necessary to decide whether or not to realize a batch of a drug for sale.
4. To ensure the existence of documented evidence, trace ability, and to provide records and an audit trail that will permit investigation.
5. It ensures the availability of the data needed for validation, review and statistical analysis.
The design and use of document depend upon the manufacturer. (WHO, Vol.2)
SCOPE
Good documentation encompasses practically all the aspect of pharmaceutical production :
1. Building and premises: installation , validation , cleaning and maintenance
2. Personnel : Training, hygiene etc
3. Equipment : installation , calibration , validation , maintenance , cleaning
4. Materials: specification, testing, ware-housing, use, rejection/disposal.
5. Processing: individual steps in the process of manufacturing including controls thereof.
6. Finished goods: specifications, testing, storage, distribution, and rejection/disposal.
Complaints: investigation, actions (including recall, if necessary). (OPPI Guideline)
CHARACTERISTIC OF DOCUMENT
For effective use of documents, they should be designed and prepared with utmost care
Each document shall:
(i) Have a clear title.
(ii) Have an identification number.
(iii) Be approved by authorized person.
(iv) Have the date of issue
(v) Have a due date of revision.
(vi) List to whom it has been issued.
Where the documents carry instructions (e.g. batch processing)
i. The instructions shall be precise and not ambiguous.
ii. They shall be for each individual step and not combined.
E.g. Weigh the materials; charge the weighed materials into the blend
iii. Instructions shall be in imperative mood.
Where entry of any data (e.g. temperature, weight) is expected to be made by the person using the document:
i. Sufficient space shall be provided for making the entry.
ii. Heading shall clearly indicate what is to be entered, and who is responsible.
iii. All entries shall be in ink.
iv. All entries shall be clear and legible.
v. Person making the entries shall confirm the entry by initialing/signing the same.
vi. An error in entry shall be so corrected that the original (wrong) entry is not lost. such correction shall also be initialed and dated. Where necessary, reason for correction shall also be recorded, initialed and dated.
Documentation system should provide for a periodic review, and revision, if necessary, of any document, or part thereof.
Such revised versions shall also be approved by the authorized persons.
Updated/revised versions shall also be superseding the previous edition, and the document shall clearly indicate this.
Outdate/superseded document shall be immediately removed from active use, and copy retained only for reference. If documentation is through electronic data processing system (computerized system) there shall be adequate, reliable systems in place:
1. To check and ensure correctness of data.
2. To record changes (addition/deletion)
3. That meets other regulation requirement, if any. (Kate McCornick)
For implementing efficient documentation practices, which meet, full GLP/GMP/ISO and FDA requirements. Here is a hint from the “documents” model, which lists out the areas required for GMP document implementation:
D= Design, development, deviations, dossiers and Drug Master Files for regulated markets, distribution records
O= Operational procedures/techniques/methods, Out of specifications (OOS), Out of trend (OOT)
C= Cleaning, calibration, controls, complaints, containers and closures, contamination and change control
U= User requirement specifications, utilities like water systems, HVAC, AHU etc.
M= Man, materials, machines, methods, maintenance, manufacturing operations and controls, monitoring, master formula, manuals (quality, safety and environment), medical records
E= Engineering control and practices, Environment control, Equipment qualification documents
N= Non-routine activities, New products and substances
T = Technology transfer, training, testing, Trend analysis, Technical dossiers
S= SOPs, safety practices, sanitation, storage, self-inspection, standardization, supplier qualification, specifications and standard test procedures and site master file. (Quality Assurance)
MASTER FORMULA RECORD
Master formula record is a product specific document compiled, checked, authorized and approved by competent technical personnel from different. But interlinked, functions such as development, production, packaging and quality control as necessary and appropriate.
As with any other documentation master formula record shall also be open for review. Changes, if any shall also be approved by designated persons responsible for production and quality control.
Master formula record shall;
a. Give patent/proprietary name of the product, and its strength.
b. Give pharmacopoeial/generic name of the product, and its strength.
c. Give dosage form (e.g. tablet, ampoule) and physical characteristics of the product.
d. Give sufficient, detailed information of product pack and primary packaging materials.
e. Give identity, quality and quantity of every ingredient, including overages/assay value based quantities, if any, irrespective of whether, or not, the material.
1) Is an active drug substance in the formulation/product,
2) Is used as a pharmaceutical aid (excipient).
3) Appears, or is detected/tested in the final product.
f. Briefly describe all the raw materials
g. Give broad outlines of the process of manufacture (as a flow-chart, for example).
h. Give brief description of equipment/ machinery used for manufacturing the product.
i. Give step-wise manufacturing process.
j. Give theoretical and practical (expected) yields at different stages of manufacture.
k. Bring out in sufficient details precautions to be taken during manufacturing to ensure birth product quality and personnel safety.
l. Give all analytical controls, including limits thereof, applicable to the finished product.
m. Give stability test results covering the assigned shelf life..
n. Have a ‘product history’ data giving references in manufacturing/packaging introduced over the year.
o. (Preferably) contain samples of printed packaging components.
A format, which may be used as guideline for preparing master formula record , is as below
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE

Specification of Raw Material:
Name of Raw Material Specification
Specifications of container, closure, labeling and packaging Materials:
Name of item Specification
Weighment sheet:
Dispensing instructions:
List of Equipment and Machinery (may be appended as annexure):
Manufacturing Instruction including in-process controls to be exercised
Packaging Material Requirements ( requisition and issue formats);
Packaging instruction:
Precautions to be taken
Finished product specification
Expiry date
Review due on (Sharma P.P)
BATCH MANUFACTURING RECORD
Batch manufacturing record is a product and batch specific document designed to give a complete and reliable picture of the manufacturing history of each batch of every product.
Batch manufacturing record shall be essentially based on the master formula record and shall be compiled, checked, approved and authorized by competent technical person responsible for production and quality control. Photo reproduction, or such other system (e.g. computer printouts) shall be preferred to avoid transcription errors provided, however, there are adequate safeguards to prevent unauthorized re-production.
· Name of the product
· Batch number
· Date of commencement & completion of significant intermediate stage
· Name of the person responsible for each stage of production
· Initials of operators who carried out significant processes and initial of persons who checked, wherever applicable
· Quantity, batch number, quality control report number of each ingredient actually weighed and amount of any recovered material added
· In-process controls carried out their results and signature of person who performed
· Theoretical yield & actual yield at appropriate stage of production together with explanation, if variation beyond expectation observed
· Authorization of any deviation, if made
In addition to the mandatory ( schedule U, drugs & cosmetics rules, 1945 ) requirement, the batch manufacturing record should also include; (D.H. Shah, Sharma P.P)
FOR TABLETS
M/S (name & address of the company)
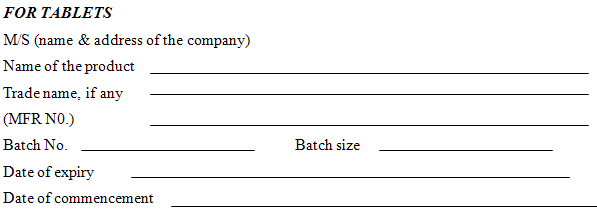
|
s.no. |
Ingredients In order of mixing |
Standards |
Q/C report No. |
Label Claim |
Quantity required |
Quantity Actually Used |
Remarks % Overages |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
|
|
|
|
|
|
|
|

MIXING
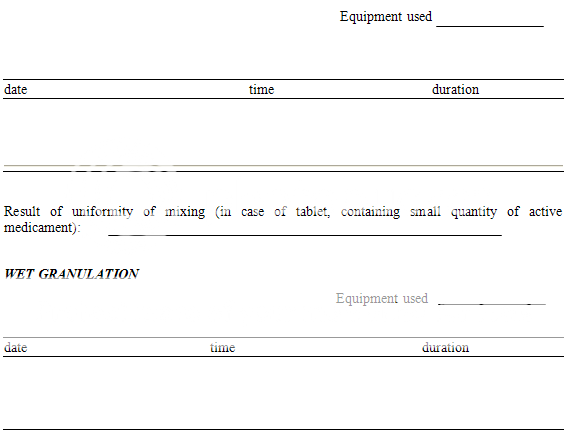
DRYING

|
Date |
Start Time |
Close Time |
Name of persons responsible for |
||
|
Stripping |
Other packg. |
||||
|
Stripping |
Checking |
Counting & filling In boxes |
|||
|
|
|
|
|
|
|
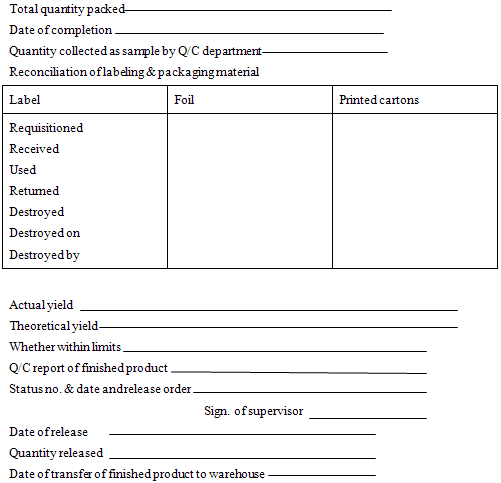
Total quantity packed
Date of completion
Quantity collected as sample by Q/C department
Reconciliation of labeling & packaging material
|
Label |
Foil |
Printed cartons |
|
Requisitioned Received Used Returned Destroyed Destroyed on Destroyed by |
|
|
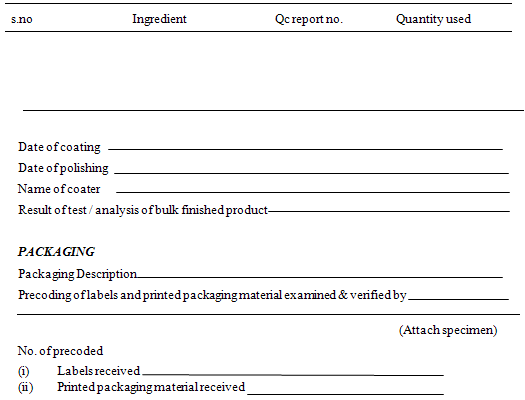
BATCH PACKAGING RECORDS
In fact, batch packaging record is a part of batch process record. These records are based on packaging instruction. One important operation that should be carried out before packaging operation is line purging. WHO guidelines require that following information should be recorded at the time of each action
· The name , batch number & quantity of the bulk finished product to be packed
· Theoretical yield & actual yield and reconciliation
· The date and time of the packaging operations
· The name of the responsible person carrying out the packaging operations
· Initial of the operators of the different significance step
· In-process control checks & the checks made for identity & conformity with the packaging instruction
· Detail of packaging operation like equipment and the packaging lines used, when necessary, the instruction for keeping the product unpacked or a record of unpacked product sent back to storage area
· Sample of printed packaging material used, bearing the batch number, expiry date and any additional over printing;
· In any case of problem, if any deviation made, written authorization for the same;
· Quantity along with identification of different packaging materials issued, used, destroyed and/ or returned to store and reconciliation. (Sharma P.P)
STANDARD OPERATING PROCEDURES (SOP)
The terms standard operating procedure and standing operating procedure, both abbreviated by the initialism, SOP, occur in a variety of different contexts, such as healthcare, education, industry, the military, etc.
One of the important activities in the implementation GMPis preparation of SOPS. One may very well ask why should there be SOPS. One of the objectives of GMPS is consistency in quality. Consistency in quality can be achieved by minimizing sources of quality variation. SOPS can be defined as written documents specifying the procedure that must be followed to carry out operation. One of the purposes of SOPS is to reduce the introduction of errors and variation in the operation. The other purpose of sops is of historical perspective i.e. how an operation was carried out.
1) An SOP is a written document or instruction detailing all steps and activities of a process or procedure. These should be carried outwithout any deviation or modification to guarantee the expected outcome. Any modification or deviation from a given SOP should be thoroughly investigated and outcomes of the investigation documented according the internal deviation procedure. (wikipedia.org/wiki/Standing_operating_procedure)
All quality impacting processes and procedures should be laid outin Standard Operating Procedures (SOPs).
Benefits of SOP
1. To provide people with all the safety, health, environmental and operational information necessary to perform a job properly. Placing value only on production while ignoring safety, health and environment is costly in the long run. It is better to train employees in all aspects of doing a job than to face accidents, fines and litigation later.
2. To ensure that production operations are performed consistently to maintain quality control of processes and products. Consumers, from individuals to companies, want products of consistent quality and specifications. SOPs specify job steps that help standardize products and therefore quality.
3. To ensure that processes continue uninterrupted and are completed on a prescribed schedule. By following SOPs, you help ensure against process shut-downs caused by equipment failure or other facility damage.
4. To ensure that no failures occur in manufacturing and other processes that would harm anyone in the surrounding community. Following health and environmental steps in SOPs ensures against spills and emissions that threaten plant neighbors and create community outrage.
5. To ensure that approved procedures are followed in compliance with company and government regulations. Well-written SOPs help ensure that government regulations are satisfied. They also demonstrate a company's good-faith intention to operate properly. Failure to write and use good SOPs only signals government regulators that your company is not serious about compliance.
6. To serve as a training document for teaching users about the process for which the SOP was written.Thorough SOPs can be used as the basis for providing standardized training for employees who are new to a particular job and for those who need re-training.
7. To serve as a checklist for co-workers who observe job performance to reinforce proper performance. The process of actively caring about fellow workers involves one worker coaching another in all aspects of proper job performance. When the proper procedures are outlined in a good SOP, any co-worker can coach another to help improve work skills.
8. To serve as a checklist for auditors. Auditing job performance is a process similar to observation mentioned in the previous item only it usually involves record keeping. SOPs should serve as a strong basis when detailed audit checklists are developed.
9. To serve as an historical record of the how, why and when of steps in an existing process so there is a factual basis for revising those steps when a process or equipment are changed. As people move from job to job within and between companies, unwritten knowledge and skills disappear from the workplace. Properly maintained written SOPs can chronicle the best knowledge that can serve new workers when older ones move on.
10. To serve as an explanation of steps in a process so they can be reviewed in accident investigations. Although accidents are unfortunate, view them as opportunities to learn how to improve conditions. A good SOP gives you a basis from which to being investigating accidents.
(pharmainfo.net/reviews/standard-operating-procedures-sop-back-bone-pharmaceutical-industries)
Some guidelines are given below to prepare SOPS.
(i) Give a clear & descriptive title to each SOP.
(ii) Provide sufficient details. The SOP should meet the need of an individual, all the same, it should be general enough for more than one user.
(iii) Flexibility should be written in the SOP wherever appropriate but it should not be made too general for, it may be useless in meeting its intended purpose
(iv) Organize SOPs according to order of sequence of events involved in performing the operation. Write the text in straight forward and easy to follow manner.
(v) After drafting SOP, use it in performing the operation to ensure that it has sufficient details to perform the operation in intended manner.
(vi) Take into account the instructions from the manufacturer of the equipment which is employed in performing the operation while drafting SOP.
(vii) Indicate total number of pages so that user is certain that he is performing the complete operation.
(viii) Indicate the effective date of the SOP.
The best way to prepare SOPs is to involve at least one person from each work area. The person selected should be asked to write down the procedure of the operation with details and the precautions to be taken. The written down procedure should be discussed by a group of persons intimately connected with the operation. Modifications, if any, should be made. This should be handed over to the person who has been designated as coordinator. The coordinator should rewrite it is needed to bring uniformity in style & format.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
It will be advisable to first prepare SOP for SOP. The following explanatory notes will be useful in preparing SOP for SOP
· Design a format for SOP. This will maintain uniformity in writing SOPs
· Device a system of assigning number to SOP. This may include a code for the department, activity to which it is related & version. For example, SOP for equipment cleaning in production department may be numbered as PR/EC/01/V1 or PR.EC01.V!
· Device a date format. Date format could be: DD/MM/YY or DD/MM/YYYY.
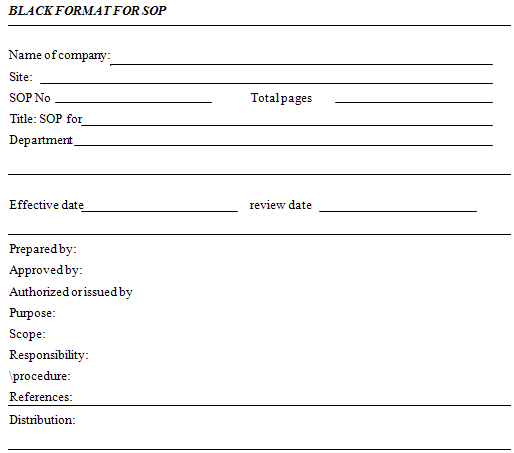
· Define elements of SOP, usually the following elements are included in a SOP:
- SOP number
- Title of SOP
- Department
- Prepared by, approved by, authorized or issued by:
- Purpose/object
- Scope
- Responsibility
- Procedure
- References
- Distribution
A blank format given on next page may be seen for guidance. It will be useful if a history page has record of revision to that SOP. This page may contain the following information:
- Version number
- Reasons for revision
- Nature of revision
- revised by
- Retrieval and distribution of obsolete copies (Sharma P.P)
DOCUMENT REQUIRED AS PER ICH GUIDELINE:
Documentation System and Specifications
1. All documents related to the manufacture of intermediates or APIs should be prepared, reviewed, approved and distributed according to written procedures. Such documents can be in paper or electronic form.
2. The issuance, revision, superseding and withdrawal of all documents should be controlled with maintenance of revision histories.
3. A procedure should be established for retaining all appropriate documents (e.g., development history reports, scale-up reports, technical transfer reports, process validation reports, training records, production records, control records, and distribution records). The retention periods for these documents should be specified.
4. All production, control, and distribution records should be retained for at least 1 year after the expiry date of the batch. For APIs with retest dates, records should be retained for at least 3 years after the batch is completely distributed.
5. When entries are made in records, these should be made indelibly in spaces provided for such entries, directly after performing the activities, and should identify the person making the entry. Corrections to entries should be dated and signed and leave the original entry still readable.
6. During the retention period, originals or copies of records should be readily available at the establishment where the activities described in such records occurred. Records that can be promptly retrieved from another location by electronic or other means are acceptable.
7. Specifications, instructions, procedures, and records can be retained either as originals or as true copies such as photocopies, microfilm, microfiche, or other accurate reproductions of the original records. Where reduction techniques such as microfilming or electronic records are used, suitable retrieval equipment and a means to produce a hard copy should be readily available.
8. Specifications should be established and documented for raw materials, intermediates where necessary, APIs, and labeling and packaging materials. In addition, specifications may be appropriate for certain other materials, such as process aids, gaskets, or other materials used during the production of intermediates or APIs that could critically impact on quality. Acceptance criteria should be established and documented for in-process controls.
9. If electronic signatures are used on documents, they should be authenticated and secure.
Equipment Cleaning and Use Record
1. Records of major equipment use, cleaning, sanitization and/or sterilization and maintenance should show the date, time (if appropriate), product, and batch number of each batch processed in the equipment, and the person who performed the cleaning and maintenance.
2. If equipment is dedicated to manufacturing one intermediate or API, then individual equipment records are not necessary if batches of the intermediate or API follow in traceable sequence. In cases where dedicated equipment is employed, the records of cleaning, maintenance, and use can be part of the batch record or maintained separately.
Records of Raw Materials, Intermediates, API Labeling and Packaging Materials
1. Records should be maintained including:
- The name of the manufacturer, identity and quantity of each shipment of each batch of raw materials, intermediates or labeling and packaging materials for API's; the name of the supplier; the supplier's control number(s), if known, or other identification number; the number allocated on receipt; and the date of receipt;
- The results of any test or examination performed and the conclusions derived from this;
- Records tracing the use of materials;
- Documentation of the examination and review of API labeling and packaging materials for conformity with established specifications; and
- The final decision regarding rejected raw materials, intermediates or API labeling and packaging materials.
2. Master (approved) labels should be maintained for comparison to issued labels.
Master Production Instructions (Master Production and Control Records)
1. To ensure uniformity from batch to batch, master production instructions for each intermediate and API should be prepared, dated, and signed by one person and independently checked, dated, and signed by a person in the quality unit(s).
2. Master production instructions should include:
- The name of the intermediate or API being manufactured and an identifying document reference code, if applicable;
- A complete list of raw materials and intermediates designated by names or codes sufficiently specific to identify any special quality characteristics;
- An accurate statement of the quantity or ratio of each raw material or intermediate to be used, including the unit of measure. Where the quantity is not fixed, the calculation for each batch size or rate of production should be included. Variations to quantities should be included where they are justified;
- The production location and major production equipment to be used;
- Detailed production instructions, including the:
(i) sequences to be followed,
(ii) ranges of process parameters to be used,
(iii) sampling instructions and in-process controls with their acceptance criteria, where appropriate,
(iv)time limits for completion of individual processing steps and/or the total process, where appropriate; and
(v) expected yield ranges at appropriate phases of processing or time;
- Where appropriate, special notations and precautions to be followed, or cross-references to these; and
- The instructions for storage of the intermediate or API to assure its suitability for use, including the labeling and packaging materials and special storage conditions with time limits, where appropriate.
Batch Production Records (Batch Production and Control Records)
1. Batch production records should be prepared for each intermediate and API and should include complete information relating to the production and control of each batch. The batch production record should be checked before issuance to assure that it is the correct version and a legible accurate reproduction of the appropriate master production instruction. If the batch production record is produced from a separate part of the master document, that document should include a reference to the current master production instruction being used.
2. These records should be numbered with a unique batch or identification number, dated and signed when issued. In continuous production, the product code together with the date and time can serve as the unique identifier until the final number is allocated.
3. Documentation of completion of each significant step in the batch production records (batch production and control records) should include:
- Dates and, when appropriate, times;
- Identity of major equipment (e.g., reactors, driers, mills, etc.) used;
- Specific identification of each batch, including weights, measures, and batch numbers of raw materials, intermediates, or any reprocessed materials used during manufacturing;
- Actual results recorded for critical process parameters;
- Any sampling performed;
- Signatures of the persons performing and directly supervising or checking each critical step in the operation;
- In-process and laboratory test results;
- Actual yield at appropriate phases or times;
- Description of packaging and label for intermediate or API;
- Representative label of API or intermediate if made commercially available;
- Any deviation noted, its evaluation, investigation conducted (if appropriate) or reference to that investigation if stored separately; and
- Results of release testing.
4. Written procedures should be established and followed for investigating critical deviations or the failure of a batch of intermediate or API to meet specifications. The investigation should extend to other batches that may have been associated with the specific failure or deviation.
Laboratory Control Records
1. Laboratory control records should include complete data derived from all tests conducted to ensure compliance with established specifications and standards, including examinations and assays, as follows:
- A description of samples received for testing, including the material name or source, batch number or other distinctive code, date sample was taken, and, where appropriate, the quantity and date the sample was received for testing;
- A statement of or reference to each test method used;
- A statement of the weight or measure of sample used for each test as described by the method; data on or cross-reference to the preparation and testing of reference standards, reagents and standard solutions;
- A complete record of all raw data generated during each test, in addition to graphs, charts, and spectra from laboratory instrumentation, properly identified to show the specific material and batch tested;
- A record of all calculations performed in connection with the test, including, for example, units of measure, conversion factors, and equivalency factors;
- A statement of the test results and how they compare with established acceptance criteria;
- The signature of the person who performed each test and the date(s) the tests were performed; and
- The date and signature of a second person showing that the original records have been reviewed for accuracy, completeness, and compliance with established standards.
2. Complete records should also be maintained for:
- Any modifications to an established analytical method;
- Periodic calibration of laboratory instruments, apparatus, gauges, and recording devices;
- All stability testing performed on APIs; and
- Out-of-specification (OOS) investigations.
Batch Production Record Review
1. Written procedures should be established and followed for the review and approval of batch production and laboratory control records, including packaging and labeling, to determine compliance of the intermediate or API with established specifications before a batch is released or distributed.
2. Batch production and laboratory control records of critical process steps should be reviewed and approved by the quality unit(s) before an API batch is released or distributed. Production and laboratory control records of non-critical process steps can be reviewed by qualified production personnel or other units following procedures approved by the quality unit(s).
3. All deviation, investigation, and OOS reports should be reviewed as part of the batch record review before the batch is released.
4. The quality unit(s) can delegate to the production unit the responsibility and authority for release of intermediates, except for those shipped outside the control of the manufacturing company. (ICH Guideline)
DOCUMENT REQUIRED AS PER WHO GUIDELINE:-
Principle: - Good documentation is an essential part of the quality assurance system and, as such, should exit for all aspects of GMP. Its aims are to define the specifications and procedures for all materials and method of manufactured and control, to ensure that all personal concern with manufacture know what to do and when to do it, to ensure that authorized persons have all the information necessary to decide whether or not to realize a batch of a drug for sale, to ensure the existence of documented evidence, trace ability, and to provide records and an audit trail that will permit investigation. It ensures the availability of the data needed for validation, review and statistical analysis. The design and use of document depend upon the manufacturer
General information
· Documents should be designed, prepared, reviewed and distributed with care. They should comply with the relevant part of the manufacturing and marketing authorizations.
· Documents should be approved, signed and dated by the appropriate responsible persons. No document should be changed without authorization and approval.
· Documents should have unambiguous contents: the title, nature and purpose should be clearly stated. They should be laid out in an orderly fashion and be easy to check. Reproduced documents should be easy to check. Reproduced documents should be clear and legible. The reproduction of working documents from master documents must not allow any error to be introduced through the reproduction process.
· Documents should be regularly reviewed and kept up to date when a document has been revised, a system should exist to prevent inadvertent use of the superseded version superseded documents should be retained for a specific period of time.
· Documents should not be hand written .Where documents require the entry should be clear, legible and indelible. Sufficient space should be provided for such entries.
· Any alteration made to a document should be signed and dated: the alteration should permit the reading of the original information. Where appropriate, the reason for the alteration should be recorded.
· Records should be made or completed when any action is taken and in such a way that all significant activities concerning the manufacture of pharmaceutical products are traceable. Records should be retained for at least one year after the expiry date of the finished product.
· Data (and records for storage) may be recorded by electronic data processing systems or by photographic or other reliable means. Master formulae and detailed standard operating procedures relating to the system in use should be available and the accuracy of the records should be checked. If documentation is handled by electronic data-processing methods. Only authorized persons should be able to enter or modify data in the computer, and there should be a record of changes and deletions: access should be restricted by passwords or other means and the entry of critical data should be independently checked. Batch records stored electronically should be protected by back-up transfer on magnetic tape, microfilm, paper print-outs or other means. It is particularly important that, during the period of retention, the data are readily available.
· Each specification should be approved, signed and dated, and maintained by quality control, quality assurance unit or documentation center. Specifications for starting materials, intermediates, and bulk, finished products and packaging materials.
Standard operating procedures, specifications and master formulae
Descriptive documents give instructions on how to perform a procedure or a study, or give a description of specifications. The instruction type documents are: standard Operating procedures (SOP); protocols (for validation studies, stability studies, safety studies); and master formulae (manufacturing instructions). Each of these gives instruction on how to perform specific procedures. Specifications describe the required characteristics or composition of a product or material or test. These kinds of documents provide the specific details defining the quality of incoming materials, the quality of the production environment, the quality of the production and control process, and the quality of the final product.
Forms for recording data
Another type of documentation is the form used for recording data as it is taken during the performance of tasks, tests, or events. These are forms (datasheets, or data record forms), reports, batch processing records, and equipment log books. These documents provide the evidence that the raw materials, facility environment, the production process, and the final product consistently meet the established quality requirements.
Identification numbers
There are also the identification systems or codes devised to number and track both information and documents. These are SOP numbers, equipment numbers, form numbers, receiving codes, and batch/lot numbers. These numbering systems should be designed so that procedures, processes and materials can be traced throughout the data records.(Gillian Chaloner-Larsson)
Specification for starting & packaging material
Specifications for starting, primary and printed packaging materials should include, if applicable:
1. A description of the materials. Including:
(a) The designated name and internal code reference:
(b) The reference, if any, to a pharmacopoeial monograph.
(c) Qualities and quantitative requirement with acceptance limit;
2. Depending on the company’s practice other data may be added to the specification such as
(a) The supplier and the original producer of the materials.
(b) A specimen of printed materials
(c) Direction for sampling and testing, or a reference to procedures.
(d) Storage conditions and precautions.
(e) The maximum period of storage before re-examination
Packaging material should conform to specifications, and should be compatible with the material and/or with the drug product it contains. The material should be examined for compliance with the specification, and for defects as well as for the correctness of identity markings.
Specification for intermediate & bulk product
Specifications for intermediate and bulk products should be available. The specifications should be similar to specifications for starting materials or for finished products, as appropriate.
Specification for finished product
Specifications for finished products should include:
(a) The designated name of the product and the code reference. Where applicable
(b) The designated name (s) of the active ingredient (s) (if applicable, with the INN(s).
(c) The formula or a reference to the formula.
(d) A description of the dosage from and package details.
(e) Direction for sampling and testing or a reference to producers.
(f) The qualitative and quantitative requirement, with acceptance limits.
(g) The storage conditions and precautions, where applicable.
(h) The self-life
Master formulae and processing instructions
Formally authorized manufacturing formula and processing instructions should exist for each product and batch size to be manufactured. They are often combined in one document.
The Manufacturing formula should include:
(a) The name of the product, with a product reference code relating to its Specifications.
(b) A description of the pharmaceutical form, strength of the product and batch size.
(c) A list of all starting materials to be used, with the amount of each, described using the designed name and a reference which is unique to that material, mention should be made of any substance that may disappear in the course of processing.
(d) A statement of the expected final yield with the acceptable limits, and of relevant intermediate yield, where applicable.
The processing instructions should include:
(a) A statement of the processing location and the principal equipment to be used.
(b) The methods, or reference to the methods, to be used for preparing the critical equipment (e.g. cleaning, assembling, calibrating, sterilizing).
(c) Detailed stepwise processing instructions (e.g. checks on materials, pretreatment, sequence for adding materials, mixing time, temperatures).
(d) The instruction for any in-process controls with their limits.
(e) Where necessary, the requirement for bulk storage of the products. Including the container, labeling and special storage conditions where applicable.
(f) Any special precautions to be observed.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Packaging Instruction
Formally authorized packaging instruction should exist for each product. Pack size and type. These should normally include, or make reference to:
(a) The name of the product.
(b) A description of its pharmaceutical form, strength and, where applicable, method of application.
(c) The pack size expressed in terms of the number. Weight or volume of the product in the final container.
(d) A complete list of all the packaging materials required for a standard batch size, including quantities, sizes and types, with the code or reference number relating to the specifications for each packaging material.
(e) Where appropriate, an example or reproduction of the relevant printed packaging materials and specimens, indicating where the batch number and expiry date of the product have been marked.
(f) Special precautions to be observed, including a careful examination of the packaging area and equipment in order to ascertain the line clearance before and after packaging operations.
(g) A description of the packaging operations, including any significant subsidiary operations, and equipment to be used.
(h) Details of in-process controls with instructions for sampling and acceptance limits.
Batch Processing Record
· A batch processing record should be kept for each batch processed. It should be based on the relevant parts of the currently approved master formulae & processing instruction. The method of preparation of such records should be designed to avoid errors the record should carry the number of batch being manufactured.
· Before any processing begins, a check should be made that the equipment and work stations are clear or previous products, documents, of materials not required for the planned process, and that the equipment is clean and suitable for use. The check should be recorded.
· During processing, the following information should be recorded at the time each action is taken. And after completion the record should be dated and signed by the person responsible for the processing operations.
a) The name of the product.
b) The number of the batch being manufactured.
c) Dates and times of commencement, of significant intermediate stages, and of completion of production.
d) The name of the person responsible for each stage of production.
e) The initials of the operator (s) of different significant intermediate stages, and of completion of production.
f) The batch number and/or analytical control number and the quantity of each starting material actually weighted (including the batch number and amount of any recovered or reprocessed material added).
g) Any relevant processing operation or event and the major equipment used.
h) The in-process controls performed. The initials of the person (s) carrying them out, and the result obtained.
i) The amount of product obtained at different and pertinent stages of manufacture (yield) together with comments or explanations for significant deviations from the expected yield.
j) Notes on special problems including details, with signed authorization for any deviation from the master formula & processing instruction
Batch Packaging Records
A batch packaging record should be kept for each batch or part batch processed. It should be based on the relevant part of the approved packaging instructions, and the method of preparing such record should be designed to avoid errors. Transcribing from approved documents should be avoided.
Before any packaging operation begins, checks should be made that the equipment and work station are clear of previous products, documents or materials not required for the planned packaging operations, and that equipment is clean and suitable for use. These checks should be recorded.
The following information should be recorded at the time each action is taken, and the date and the person responsible should be clearly identified by signature or electronic password.
a) The name of the product, the batch number and the quantity of bulk product to be packed, as well as the batch number and the planned quantity of finished product that will be obtained, the quantity actually obtained and the reconciliation.
b) The date (s) and time (s) of the packaging operations
c) The name of the responsible person carrying out the packaging operation.
d) The initials of the operators of the different signification steps.
e) The checks made for identity and conformity with the packaging instructions, including the results of in-process controls.
f) Details of the packaging operations carried out, including references to equipment and the packaging lines used, and, when necessary, the instructions for keeping the product unpacked or a record of returning product that has not been packaged to the storage area.
g) Whenever possible, samples of the printed packaging materials used, including specimens bearing the approval for the printing of and regular check ( where appropriate) of the batch number expiry date, and any additional overprinting.
h) Notes on any special problem, including details of any deviation from the packaging instruction, with written authorization by an appropriate person.
i) The quantities and reference number or identification of all printed packaging materials and bulk product issued, used, destroyed or retuned to stock and the quantities of product obtained to permit an adequate reconciliation.
Standard operating procedure (SOPs)
Standard operating procedure and associated records of actions taken or, where appropriate, conclusions reached should be available for:
a) equipment assembly and validation.
b) Analytical apparatus and calibration.
c) Maintenance, cleaning and sanitization.
d) Personnel matter including qualification, training, clothing and hygiene:
e) Environment monitoring
f) Pest control
g) Complaints
h) Recalls
i) Returns
· There should be standard operating procedures and records for the receipt of each delivery of starting material and primary and printed packaging material.
The records of the receipts should include.
(a) The name of the material on the delivery note and the containers
(b) The “in-house” name and/or code of material it different from (a)
(c) The date of receipt
(d) The supplier’s name and, if possible, manufacturer’s name
(e) The manufacturer’s batch or reference number
(f) The total quantity and number of containers received.
(g) The batch number assigned after receipt
(h) Any relevant comment (e.g. state of the containers)
· There should be standard operating procedures for the internal labeling, quarantine and storage of starting materials, packaging materials and other materials, as appropriate.
· Standard operating procedures should be available for each instrument and place of equipment (e.g. use, calibration, cleaning, maintenance) and placed in close proximity to the equipment
The Other Various Analysis Records
These records includes:
· Written release and rejection record should be available for materials and products, and in particular for the release for sale of the finished product by an authorized person
· Records should be maintained of the distribution of each batch of a product in order, e.g. to facilitate the recall of the batch if necessary.
· Records should be kept for major and critical equipment, as appropriate, of any validations, calibrations, maintenance, cleaning or repair operations, including dates and the identity of the people who carried these operations out.
· The use of major and critical equipment and the areas where products have been processed should be appropriately recorded in chronological order.
· There should be written procedures assigning responsibility for cleaning and sanitation and describing in sufficient detail the cleaning schedules, methods, equipment and materials to be used and facilities and equipment to be cleaned, such written procedures should be followed .(WHO, VOl 2).
THE VARIOUS RECORDS REQUIRED AS PER THE USFDA GUIDELINE
These include the records which are come under WHO GMP as well as include the other records that are:
Device master record
Each manufacturer shall maintain device master records (DMR’s). Each manufacturer shall ensure that each DMR is prepared and approved. The DMR for each type of device shall include, or refer to the location of, the following information:
(a) Device specifications including appropriate drawings, composition, formulation, component specifications, and software specifications;
(b) Production process specifications including the appropriate equipment specifications, production methods, production procedures, and production environment specifications;
(c) Quality assurance procedures and specifications including acceptance criteria and the quality assurance equipment to be used;
(d) Packaging and labeling specifications, including methods and processes used; and
(e) Installation, maintenance, and servicing procedures and methods.
Device history record.
Each manufacturer shall maintain device history records (DHR’s). Each manufacturer shall establish and maintain procedures to ensure that DHR’s for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the DMR and the requirements of this part. The DHR shall include, or refer to the location of, the following information:
(a) The dates of manufacture;
(b) The quantity manufactured;
(c) The quantity released for distribution;
(d) The acceptance records which demonstrate the device is manufactured in accordance with the DMR;
(e) The primary identification label and labeling used for each production unit; and
(f) Any device identification(s) and control number(s) used.
Quality system record.
Each manufacturer shall maintain a quality system record (QSR). The QSR shall include, or refer to the location of, procedures and the documentation of activities required by this part Each manufacturer shall ensure that the QSR is prepared and approved.
Complaint files
(a) Each manufacturer shall maintain complaint files. Each manufacturer shall establish and maintain procedures for receiving, reviewing, and evaluating complaints by a formally designated unit. Such procedures shall ensure that:
(1) All complaints are processed in a uniform and timely manner;
(2) Oral complaints are documented upon receipt; and
(3) Complaints are evaluated to determine whether the complaint represents an event which is required to be reported to FDA.
(b) Each manufacturer shall review and evaluate all complaints to determine whether an investigation is necessary. When no investigation is made, the manufacturer shall maintain a record that includes the reason no investigation was made and the name of the individual responsible for the decision not to investigate.
(c) Any complaint involving the possible failure of a device, labeling, or packaging to meet any of its specifications shall be reviewed, evaluated, and investigated, unless such investigation has already been performed for a similar complaint and another investigation is not necessary.
(d) Any complaint that represents an event which must be reported to FDA shall be promptly reviewed, evaluated, and investigated by a designated individual(s) and shall be maintained in a separate portion of the complaint files or otherwise clearly identified.
(e) records of investigation under this paragraph shall include a determination of:
(1) Whether the device failed to meet specifications;
(2) Whether the device was being used for treatment or diagnosis; and
(3) The relationship, if any, of the device to the reported incident or adverse event.
(e) When an investigation is made under this section, a record of the investigation shall be maintained by the formally designated unit identified in paragraph (a) of this section. The record of investigation shall include:
(1) The name of the device;
(2) The date the complaint was received;
(3) Any device identification(s) and control number(s) used;
(4) The name, address, and phone number of the complainant;
(5) The nature and details of the complaint;
(6) The dates and results of the investigation;
(7) Any corrective action taken; and
(8) Any reply to the complainant.
(f) When the manufacturer’s formally designated complaint unit is located at a site separate from the manufacturing establishment, the investigated complaint(s) and the record(s) of M investigation shall be reasonably accessible to the manufacturing establishment.
(g) If a manufacturer’s formally designated complaint unit is located outside of the United States, records required by this section shall be reasonably accessible in the United States at either:
(1) A location in the United States where the manufacturer’s records are regularly kept; or
(2) The location of the initial distributor. ((fda.gov))
DOCUMENTATION ACCORDING TO INSPECTION AND STANDARDS DIVISION OF THE MEDICINE AND HEALTHCARE PRODUCTS REGULATORY AGENCY
Principle
Good documentation constitutes an essential part of the quality assurance system. Clearly written documentation prevents errors from spoken communication and permits tracing of batch history. Specifications, Manufacturing Formulae and instructions, procedures, and records must be free from errors and available in writing. The legibility of documents is of paramount importance.
General
1. Specificationsdescribe in detail the requirements with which the products or materials used or obtained during manufacture have to conform. They serve as a basis for quality evaluation.
Manufacturing Formulae, Processing and Packaging Instructionsstate all the starting materials used and lay down all processing and packaging operations.
Procedures give directions for performing certain operations e.g. cleaning, clothing, environmental control, sampling, testing, equipment operations.
Records provide a history of each batch of product, including its distribution, and also of all other relevant circumstances pertinent for the quality of the final product.
2. Documents should be designed, prepared, reviewed and distributed with care. They should comply with the relevant parts of the manufacturing and marketing authorization dossiers.
3. Documents should be approved, signed and dated by appropriate and authorized persons.
4. Documents should have unambiguous contents; title, nature and purpose should be clearly stated. They should be laid out in an orderly fashion and be easy to check. Reproduced documents should be clear and legible. The reproduction of working documents from master documents must not allow any error to be introduced through the reproduction process.
5. Documents should be regularly reviewed and kept up-to-date. When a document has been revised, systems should be operated to prevent inadvertent use of superseded documents.
6. Documents should not be hand-written; although, where documents require the entry of data, these entries may be made in clear, legible, indelible handwriting. Sufficient space should be provided for such entries.
7. Any alteration made to the entry on a document should be signed and dated; the alteration should permit the reading of the original information. Where appropriate, the reason for the alteration should be recorded.
8. The records should be made or completed at the time each action is taken and in such a way that all significant activities concerning the manufacture of medicinal products are traceable. They should be retained for at least one year after the expiry date of the finished product.
9. Data may be recorded by electronic data processing systems, photographic or other reliable means, but detailed procedures relating to the system in use should be available and the accuracy of the records should be checked. If documentation is handled by electronic data processing methods, only authorized persons should be able to enter or modify data in the computer and there should be a record of changes and deletions; access should be restricted by passwords or other means and the result of entry of critical data should be independently checked. Batch records electronically stored should be protected by back-up transfer on magnetic tape, microfilm, paper or other means. It is particularly important that the data are readily available throughout the period of retention.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Documents required
Specifications
1. There should be appropriately authorized and dated specifications for starting and packaging materials, and finished products; where appropriate, they should be also available for intermediate or bulk products.
Specifications for starting and packaging materials
2. Specifications for starting and primary or printed packaging materials should include, if applicable:
a) A description of the materials, including:
· ?the designated name and the internal code reference;
· ?the reference, if any, to a pharmacopoeial monograph;
· ?the approved suppliers and, if possible, the original producer of the products;
· ?a specimen of printed materials;
b) Directions for sampling and testing or reference to procedures;
c) Qualitative and quantitative requirements with acceptance limits;
d) Storage conditions and precautions;
e) The maximum period of storage before re-examination.
Specifications for intermediate and bulk products
3. Specifications for intermediate and bulk products should be available if these are purchased or dispatched, or if data obtained from intermediate products are used for the evaluation of the finished product. The specifications should be similar to specifications for starting materials or for finished products, as appropriate.
Specifications for finished products
4. Specifications for finished products should include:
a) The designated name of the product and the code reference where applicable;
b) The formula or a reference to;
c) A description of the pharmaceutical form and package details;
d) Directions for sampling and testing or a reference to procedures;
e) The qualitative and quantitative requirements, with the acceptance limits;
f) The storage conditions and any special handling precautions, where applicable;
g) The shelf-life.
Manufacturing formula and processing instructions
Formally authorized Manufacturing Formula and Processing Instructions should exist for each product and batch size to be manufactured. They are often combined in one document. The Manufacturing Formula should include:
a) The name of the product, with a product reference code relating to its specification;
b) A description of the pharmaceutical form, strength of the product and batch size;
c) A list of all starting materials to be used, with the amount of each, described using the designated name and a reference which is unique to that material; mention should be made of any substance that may disappear in the course of processing;
d) A statement of the expected final yield with the acceptable limits, and of relevant intermediate yields, where applicable.
The Processing Instructions should include:
a) A statement of the processing location and the principal equipment to be used;
b) The methods, or reference to the methods, to be used for preparing the critical equipment (e.g. cleaning, assembling, calibrating, sterilizing);
c) Detailed stepwise processing instructions (e.g. checks on materials, pretreatments, sequence for adding materials, mixing times, temperatures);
d) The instructions for any in-process controls with their limits;
e) Where necessary, the requirements for bulk storage of the products; including the container, labeling and special storage conditions where applicable;
f) Any special precautions to be observed.
Packaging instructions
There should be formally authorized Packaging Instructions for each product for pack size and type. These should normally include, or have a reference to, the following:
a) Name of the product;
b) Description of its pharmaceutical form, and strength where applicable;
c) The pack size expressed in terms of the number, weight or volume of the product in the final container;
d) A complete list of all the packaging materials required for a standard batch size, including quantities, sizes and types, with the code or reference number relating to the specifications of each packaging material;
e) Where appropriate, an example or reproduction of the relevant printed packaging materials, and specimens indicating where to apply batch number references, and shelf-life of the product;
f) Special precautions to be observed, including a careful examination of the area and equipment in order to ascertain the line clearance before operations begin;
g) A description of the packaging operation, including any significant subsidiary operations, and equipment to be used;
h) Details of in-process controls with instructions for sampling and acceptance limits.
Batch processing records
A Batch Processing Record should be kept for each batch processed. It should be based on the relevant parts of the currently approved Manufacturing Formula and Processing Instructions. The method of preparation of such records should be designed to avoid transcription errors. The record should carry the number of the batch being manufactured.
Before any processing begins, there should be recorded checks that the equipment and work station are clear of previous products, documents or materials not required for the planned process, and that equipment is clean and suitable for use. During processing, the following information should be recorded at the time each action is taken and, after completion, the record should be dated and signed in agreement by the person responsible for the processing operations:
a) The name of the product;
b) Dates and times of commencement, of significant intermediate stages and of completion of production;
c) Name of the person responsible for each stage of production;
d) Initials of the operator of different significant steps of production and, where appropriate, of the person who checked each of these operations (e.g. weighing);
e) The batch number and/or analytical control number as well as the quantities of each starting material actually weighed (including the batch number and amount of any recovered or reprocessed material added);
f) Any relevant processing operation or event and major equipment used;
g) A record of the in-process controls and the initials of the person(s) carrying them out, and the results obtained;
h) The amount of product yield obtained at different and pertinent stages of manufacture;
i) Notes on special problems including details, with signed authorization for any deviation from the Manufacturing Formula and Processing Instructions.
Batch packaging records
A Batch Packaging Record should be kept for each batch or part batch processed. It should be based on the relevant parts of the Packaging Instructions and the method of preparation of such records should be designed to avoid transcription errors. The record should carry the batch number and the quantity of bulk product to be packed, as well as the batch number and the planned quantity of finished product that will be obtained.
Before any packaging operation begins, there should be recorded checks that the equipment and work station are clear of previous products, documents or materials not required for the planned packaging operations, and that equipment is clean and suitable for use.
The following information should be entered at the time each action is taken and, after completion, the record should be dated and signed in agreement by the person(s) responsible for the packaging operations:
a) The name of the product;
b) The date(s) and times of the packaging operations;
c) The name of the responsible person carrying out the packaging operation;
d) The initials of the operators of the different significant steps;
e) Records of checks for identity and conformity with the Packaging Instructions including the results of in-process controls;
f) Details of the packaging operations carried out, including references to equipment and the packaging lines used;
g) Whenever possible, samples of printed packaging materials used, including specimens of the batch coding, expiry dating and any additional overprinting;
h) Notes on any special problems or unusual events including details with signed authorization for any deviation from the Manufacturing Formula and Processing Instructions;
i) The quantities and reference number or identification of all printed packaging materials and bulk product issued, used, destroyed or returned to stock and the quantities of obtained product, in order to provide for an adequate reconciliation. (M.H.R.A., PIC/S GMP Guide)
GMP GUIDELINES FOR RECORDS AND REPORTS
Equipment cleaning and use log
· A written record of major equipment cleaning, maintenance (except routine maintenance such as lubrication and adjustments), and use shall be included in individual equipment logs that show the date, time, product, and lot number of each batch processed.
· If equipment is dedicated to manufacture of one product, then individual equipment logs are not required, provided that lots or batches of such product follow in numerical order and are manufactured in numerical sequence.
· In cases where dedicated equipment is employed, the records of cleaning, maintenance, and use shall be part of the batch record.
· The persons performing and double-checking the cleaning and maintenance shall date and sign or initial the log indicating that the work was performed. Entries in the log shall be in chronological order.
· This section requires written designation of which equipment is ‘‘major.’’ The intent of the regulations is not to include small items such as ladles, scoops, stirrers, and spatulas. The exclusion of ‘‘no major’’ items from the recordkeeping requirement does not, however, exclude them from the requirements that they be properly cleaned.
· Because the log is for a repetitive operation, the record may be initialed rather than signed. Note that a separate log, which may be a completely separately bound volume, or consecutive pages in a bound or loose-leaf format, or a number of individual records or logs is required for each piece of major equipment that is not dedicated to the manufacture of a single product.
· The issue of signatures and initials has involved considerable industry–FDA interaction. As new computerized technology became available it was possible to move to paperless control of manufacturing processes. These computerized controls had several advantages over manual systems:
1. More consistent control.
2. Only approved (trained) personnel could perform a process.
3. Processing could be prevented until any prior steps or checks were performed.
4. Precise recording of the times of operations were possible.
· Electronic signatures/initials frequently involve a personal password and a personal magnetic card with a secure system to manage allocation and review.
Packaging and labeling records
These records shall include the following:
(a) The identity and quantity of each shipment of each lot of components, drug product containers, closures, and labeling; the name of the supplier; the supplier’s lot number(s) if known; the receiving code as specified in and the date of receipt. The name and location of the prime manufacturer, if different from the supplier, shall be listed if known.
(b) The results of any test or examination performed including those performed as required by and the conclusions derived there from.
(c) An individual inventory record of each component, drug product container and closure and, for each component, a reconciliation of the use of each lot of such component. The inventory record shall contain sufficient information to allow determination of any batch or lot of drug product associated with the use of each component, drug product container and closure.
(d) Documentation of the examination and review of labels and labeling for conformity with established specifications.
(e) The disposition of rejected components, drug product containers, closure, and labeling.
Laboratory records
Documentation is important in all types of activities in an organization but in a laboratory it is extremely critical.
(a) Laboratory records shall include complete data derived from all tests necessary to assure compliance with established specifications and standards, including examinations and assays, as follows:
(1) A description of the sample received for testing with identification of source (that is, location from where sample was obtained), quantity, lot number or other distinctive code, date sample was taken, and date sample was received for testing.
(2) A statement of each method used in the testing of the sample. The statement shall indicate the locations of data that establish that the methods used in the testing of the sample meet proper standards of accuracy and reliability as applied to the product tested. (If the method employed is in the current revision of the United States Pharmacopeia, National Formulary, Association of Official Analytical Chemists, Book of Methods, or in other recognized standard references, or is detailed in an approved new drug application and the referenced method is not modified, a statement indicating the method and reference will suffice.)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The suitability of all testing methods used shall be verified under actual conditions of use.
(3) A statement of the weight or measure of sample used for each test where appropriate.
(4) A complete record of all data secured in the course of each test, including all graphs, charts, and spectra from laboratory instrumentation properly identified to show the specific component, drug product container, closure, in-process material, or drug product, and lot tested.
(5) A record of all calculations performed in connection with the test, including units of measure, conversion factors, and equivalency factors.
(6) A statement of the results of tests and how the results compare with established standards of identity, strength, quality, and purity for the component, drug product container, closure, in-process material, or drug product tested.
(7) The initials or signature of the person who performs each test and the date(s) the tests were performed.
(8) The initials or signature of a second person showing that the original records have been reviewed for accuracy, completeness, and compliance with established standards.
(b) Complete records shall be maintained of any modification of an established method employed in testing. Such records shall include the reason for the modification and data to verify that the modification produced results that are at least as accurate and reliable for the material being tested as the established method.
(c) Complete records shall be maintained of any testing and standardization of laboratory reference standards, reagents, and standard solutions.
(d) Complete records shall be maintained of the periodic calibration of laboratory instruments, apparatus, gauges, and recording devices and all stability testing performed as per requirement. (Sharma P.P “GLP”, D.H. Shah)
Distribution records
· Distribution records shall contain the name and strength of the product and description of the dosage form, name and address of the consignee, date and quantity shipped, and lot or control number of the drug product. For compressed medical gas products, distribution records are not required to contain lot or control numbers.
· The primary purpose of this section is to ensure that adequate data are available to access trade customers should a recall be initiated.
· The recording of lot number to each order will certainly accomplish this purpose; other approaches can achieve the same result.
· The recording of dates on which a specific lot of product commenced and ceased distribution may be used.
· All customers receiving the product between these dates could then be contacted. Obviously on the first and last days of distribution, some of the customers may have received product from the end of the previous lot or the beginning of the next lot.
Complaint files
(a) Written procedures describing the handling of all written and oral complaints regarding a drug product shall be established and followed. Such procedures shall include provisions for review by the quality control unit, of any complaint involving the possible failure of a drug product to meet any of its specifications and, for such drug products, a determination as to the need for an investigation. Such procedures shall include provisions for review to determine whether the complaint represents a serious and unexpected adverse drug experience which is required to be reported to the Food and Drug Administration.
(b) A written record of each complaint shall be maintained in a file designated for drug product complaints. The file regarding such drug product complaints shall be maintained at the establishment where the drug product involved was manufactured, processed, or packed, or such file may be maintained at another facility if the written records in such files are readily available for inspection at that other facility.
(C) Written records involving a drug product shall be maintained until at least 1 year after the expiration date of the drug product, or 1 year after the date that the complaint was received, whichever is longer. (D.H. Shah, Sandy Weinberg)
Manufacturing working formula procedure (MWFP)
Documentation of the component materials and processing steps, together with production operation specifications and equipment to be used, make up the MWFP. A working formula procedure for each batch size that is produced to attempt expansion or reduction of a batch size by manual calculation good manufacturing practice. (Leon Lachman)
DEVELOPMENT AND IMPLEMENTATION IN DOCUMENTATION:-
The traditional records management model is based on cabinets, folder, and files. This physical model was given in logical extension in the first electronic document management system, where files were placed into virtual cabinets and folders.
File room Model and Security
Security models for documents are all based on controlling who can see document, who can create or edit documents, and who can delete documents. Securing these rights is implemented at numerous levels. It is illustrative to consider these in terms of a physical library paper-based file room. First you may need proper credentials simply to get in and browse the holdings. Second, once you have gained admittance to the filing area, your ability to view certain kinds of records may depend on your job title or departmental affiliation. Third, assuming you have rights to view a specific record, you may have permission only to view the final file under observation in the file room itself, and you may not be permitted to make a copy. Finally, if you are permitted to check the document out of the file room for a limited time, you will be required to sign your name to a dated logbook.
Input–Output Model and Quality Control:-
Traditional document management rests on a very simple input-output model. An enterprise seeks to manage the storage of documents (input) in such a way that their retrieval (output) is simplified. The real goal is speedy retrieval of documents. (James Swarbrick “Encyclopedia”)
Electronic Documentation of Pharmaceutical Calibrations
Electronic documentation systems that do not require any paper were developed to overcome these disadvantages and reduce the amount of time technicians spend in complying with documentation regulations. However, electronic records do not inherently contain signatures that identify the person performing a calibration. Multifunction Calibrators, can be integrated to provide automated documentation with less human intervention. This results in fewer human errors, improved work quality, and improved efficiency that can directly affect profit. Moreover, locating the original electronic records in one database can not only reduce paper records into traceable electronic records with a history of change management, but can also turn the calibration system into a powerful repository of decision-making history that can be used to improve calibration procedures. Versatile security settings and multilevel user accounts help to ensure the security and integrity of the system and track authorized and unauthorized database actions. (ptemag.com)
Web Document Management for the Pharmaceutical Industry
To achieve automation goals, most pharmaceutical companies would do well to start by investing in a web document management solution that can be launched from the same platform as other solutions designed for the life science industries (i.e. GxP process control, quality management and quality audit solutions). The web document management software should also provide the following features and benefits:
1: A History of Successful Validation
Quality assurance professionals and other pharmaceutical professionals know the importance of reputable software validation. When searching for a web document management solution, pharmaceutical professionals should pay close attention to its validation history.
2: Speed
Let's get real. The only reason any pharmaceutical company would even consider the purchase of a web document management solution would be to save money and time on the product-to-market pathway. If any given solution does not automate and increase the speed of document change processes, document approvals, notifications and document distribution, then the solution isn't worth consideration.
3: Time Required for Installation, Implementation and Validation-Affects on ROI
Some software vendors may tout the strengths of their software and its immediate capacity for providing a healthy ROI. However, they may conveniently fail to mention that their installation, implementation and validation processes may stretch into 6 months, a year or even longer. Pharmaceutical professionals need to search for a web document management solution that provides a healthy ROI but that makes a clear statement regarding the time that will be required for installation, implementation and validation. A clear statement will allow pharmaceutical companies to make transparent decisions and effective planning for the upcoming transitions that are inevitably linked with the switch to automated document control.
4: Configurable and "Off-the-Shelf"
If pharmaceutical companies prefer an off-the-shelf web document management solution, it must still be configurable to the unique needs of every company that purchases it. Some pharmaceutical companies for instance may not apply the same steps throughout a routing or collaboration process and the web document management solution should be able to reflect that.
5: Tracking and Audit Trails
The web document management solution should also provide tracking and audit-trail features as well as sophisticated revision controls and reporting features.
6: Electronic Signature Controls
To comply with 21 CFR Part 11, pharmaceutical companies must employ electronic signature controls. A web document management software solution that automates document signings routing and collaboration is highly recommended.
7: Compatibility with Other Existing Solutions
As mentioned earlier the web document management solution should be launched from a platform that will allow for the future launch of other solutions. These solutions may include GxP process solutions such as software for deviations identification, nonconformance identification, quality audit, customer complaint handling, change control and CAPA solutions. A submissions management solution particular to the pharmaceutical industry is also highly recommended. (ezinearticles.com/)
BIBLIOGRAPHY
1) Gillian Chaloner-Larsson, Roger Anderson etal; “AWHO Guide to Good Manufacturing Practice (GMP) requirement” 1997 Part 1 Standard Operating Procedure and Master Formulae Record Global Programme For Vaccines & Immunization Ch-1211 Geneva 27, Switzerland PP 9-10
2) ICH Guideline, Good Manufacturing Practice Guide For Active Pharmaceutical Ingredients Q7,Dated 10 November 2000
3) James Swarbrick, James C. Boylan “Encyclopedia of Pharmaceutical technology” second edition, volume 3s, Marcel Dekker Series 2002 PP- 1980-1985
4) Kate McCormick, “Pharmaceutical Engineering series: Quality and Regulatory Compliance “first published-2002, page no:74-78
5) Leon Lachman, Herbert A. Lieberman/ Joseph L. Kanig. The theory & Practice of industrial pharmacy. 3rd ed. Varghese publishing house; 2003. P. 810-815.
6) Organization of Pharmaceutical Producers of india Quality Assurance Guide-2001,Vol-II
7) Orange Guide; inspection and standards division of the Medicine and Healthcare products regulatory agency. Rules and Guidance for Pharmaceutical manufacturers and distributors 2007. Chicago (London): Pharmaceutical press; 2007. P 43-47, 58-65.
8) PIC/S GMP Guide for Active Pharmaceutical Ingredients, Developed by the International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, 1 July 2004, PE007-2.
9) Quality Assurance and Regulatory Affairs, Tonira Pharma Limited. Email: jramniwas@tonira.com
10) Sandy Weinberg. Good Laboratory Practice Regulation. 3rd ed. New York: Marcel Dekker, inc; 2005. P. 12-13,42-43,50-57.
11) Shah D.H. “QA Manual” first Edition-2000,Published by Business horizons, new Delhi, page no: 167-181
12) Sharma P.P, “How to practice GLP” second Edition, Published by Vandana publication Pvt. Ltd. Delhi, page no: 275-276
13) Sharma P.P, “How to practice GMPs” fifth Edition, Published by Vandana publication Pvt. Ltd. Delhi, page no: 169-234
14) Willing Sidney H, Stoker James R. Good Manufacturing Practices for Pharmaceutical. 4th edition. New York: Marcel Dekker, inc; 2004. PP. 282-285.
15) World Health Organization, Quality Assurance of pharmaceuticals. A compendium of guidelines and related materials, Vol. 2, 2nd updated ed. Good Manufacturing Practice & Inspection. P 45-46, 70-79.
16) Calibration in the pharmaceutical industry ptemag.com
17) Standard Operating Procedure en.wikipedia.org/wiki/Standing_operating_procedure
18) Standard Operating Procedure sop-back-bone-pharmaceutical industries pharmainfo.net/reviews/standard-operating-procedures-sop-back-bone-pharmaceutical-industries
19) U.S Food and Drug administration. fda.gov/cdrh/humfac/frqsr.html
20) Web-Document-Management-For-the-Pharmaceutical-Industry ezinearticles.com/
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









