ABOUT AUTHORS:
*Naveen Kamboj1, Sadanand Maurya2, Sonia Kamboj3, Gaurav kumar Singh2
1Chandigarh College of Pharmacy, Landran, Mohali, Panjab
2Transalam Institute of Pharmaceutical Education and Reaserch, Meerut, Uttar Pradesh
3ISF college of pharmacy moga, Punjab
*kamboj.naveen21@gmail.com
ABSTRACT
The most important method for developing the very stable and suitable formulation for thermolabile as well as thermostable drug is lyophilization which works on the principle of sublimation of ice crystal from frozen material. Principle of the process through the basis of formulation, freezing, primary drying and secondary drying . In order to design optimum lyophilization process, process development scientific need to the critical properties of the formulation and how to apply to this information to process design. Lyophilized formulation not only has the advantage of better stability, but also provide easy handling i.e. shipping and storage. This article present an overview of lyophilization process, its advantage and pharmaceutical application.
REFERENCE ID: PHARMATUTOR-ART-1844
FREEZE DRYING TECHNOLOGY
Historical Overview
Drying from the frozen state is not uncommon in nature. In the winter, snow vanishes along the roads in dry cold air without melting. In Central Siberia, scientists have found the large bodies of mammoths that have been progressively freeze-dried during the past 15,000 years. In the Peruvian high plateau, the Incas reportedly stored, in their tambos, meat that had been dried in the sun at the reduced pressure of the Andes Scientific interest in freeze-drying began at the turn of the twentieth century with a publication by Bordas and d'Arsonval at the French Academy of Sciences. Following that publication, Altman and later Gersh used this technique to prepare undistorted dry samples for microscopy. Ronald Greaves, in Cambridge, UK, began his work along those lines in the 1930s by preparing dry suspensions of living bacteria. However, this technique still was only familiar to a handful of scientists in isolated laboratories.
Then came World War II. With tens of thousands of casualties on the battlefields, human plasma was in great need, and freeze-drying again entered the limelight. Thanks to Greaves in England, François Henaff in France, and Earl Flosdorf in the United States, thousands of liters of blood were processed to isolate plasma, which was then preserved by freezing and drying. As the use of lyophilization expanded, the process began to be industrialized. Loire, Stokes, Edwards, and others designed and built the first equipment for the purpose. Called “lyophilization” by Flosdorf, the process faced its first major challenge under Sir Ernst Boris Chain, who used the technique to preserve antibiotics. Given Chain’s results turned to lyophilization to prepare vaccines and, later on, to refine blood fractions. By the mid-1950s, many industries were already using freeze drying to preserve pharmaceutical and biological products, as were the physicians and surgeons who developed tissue-banking for plastic and reconstructive surgery. Drs. Hyatt, Bassett, and Meryman of the United States Navy were among the early pioneers in the field.1,2
INTRODUCTION
In Lyophilization, or freeze drying, there is a water is frozen, followed by its removal from the sample, initially by sublimation (primary drying) and then by desorption (secondary drying). In this process, the moisture content of the product is reduced to such a low level that does not support biological growth or chemical reactions which gives the stability to the formulation. This technique useful in formulation development of drugs which are thermolabile and/or unstable in aqueous medium.3,4,5
Lyophilization process is based on the principle of sublimation of ice, without entering the liquid phase. The phase diagram of water (Figure 1) represent that two phases coexist along a line under the given conditions of temperature and pressure, while at the triple point (0.0075 ?C at 0.61kPa or 610 Nm-2; 0.01 ?C at 0.00603 atm), all three phases coexist.
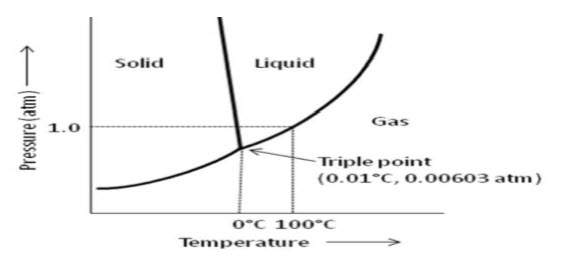
Figure 1- Phase diagram showing the triple point of water at 0.01°C, 0.00603 atm. Lyophilization is take place below the triple point.
This process is performed at temperature and pressure conditions below the triple point, to facilitate sublimation of ice. The entire process is performed at low temperature and pressure, so that useful for drying of thermolabile compounds. various important Steps involved in lyophilization process which start from sample preparation followed by freezing, primary drying and secondary drying, to obtain the final dried product with desired moisture content (Figure 2).
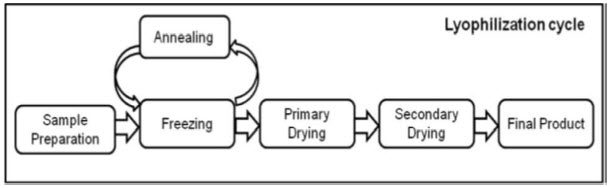
Figure 2. important Steps involved in lyophilization process from sample preparation to final product formation. Annealing is an another optional step, occasionally used to crystalline substance.
The concentration gradient of water vapour between the drying front and condenser is the driving force for removal of water during lyophilization. The vapour pressure of water increases with an increase in temperature during the primary drying. Therefore, primary drying temperature should be kept as high as possible, but below the critical process temperature, to avoid a loss of cake structure6,7,8. This critical process temperature is the collapse temperature for amorphous substance, or eutectic melt for the crystalline substance1,9,10.During freezing, ice crystals start separating out until the solution becomes maximally concentrated. On further cooling, phase separation of the solute and ice takes place. If the solute separates out in crystalline form, it is known as the eutectic temperature. In contrast, if an amorphous form is formed, the temperature is referred to as the glass transition temperature (Tg’).
Determination of this critical temperature is important for development of an optimized lyophilization cycle. During primary drying, drying temperature should not exceed the critical temperature, which otherwise leads to ‘meltback’ or ‘collapse’ phenomenon in case of crystalline or amorphous substance respectively (Figure 3).11
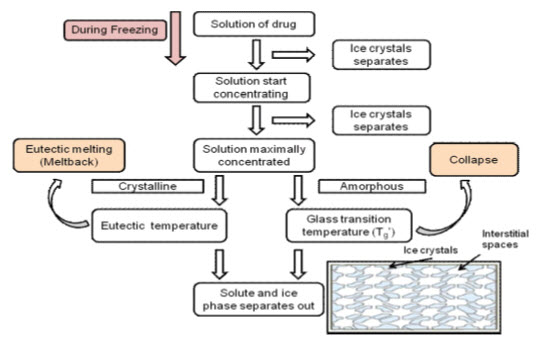
Figure 3 Flowchart showing the concept of eutectic temperature and Tg, and their importance during primary drying
ADVANTAGES-
Lyophilization process has various important advantages compared to other drying and preserving techniques.12
1-It is a ideal drying technique for heat sensitive products,
2-It can be stored at ambient temperature over a 2 year shelf life, enhanced product stability in a dry state.
3- Easy reconstitution greatly reduces weight and makes the products easier to transport, maintains food/biochemical and chemical reagent quality.
4- Reconstitution of the dried product facilitates use in emergency medicine and safe application in hospitals.
5- It is not limited to products for parenteral use, but can also be used for fast dissolving sublingual tablets. Tablets can have very low disintegration time and have great mouth feel due to fast melting effect.
6-it is much easier to achieve sterility assurance and freedom of particles than using other drying methods or handling of dry powders.
7-lyophilized products sensitive to oxidation can be stoppered and sealed within an inert atmosphere (i.e. nitrogen) to minimize detrimental effects.
DISADVANTAGES-
Although lyophilization has many advantages compared to other drying and preserving techniques it has quite a few disadvantages. It is a long and cost intensive process, requires sterile diluents for reconstitution, it should only be used when product is unstable and heat-liable and the limited amount of vials processed in each run restricts the overall production capacity. 12
MATERIAL THAT CAN BE LYOPHILIZED-
The major type of material that can be lyophilized summarized bellow-12
1-Non-living bio products this comprises the major areas of application and include:
- Enzyme, hormones, antibiotics, vitamins, blood products, inactivated vaccines etc.
- Foodstuffs where organoleptic properties are important
- Industrially useful bio-products.
- Bone and other body tissue for medical and surgical use.
2-Non-biological where the process is used to dehydrate and concentrate reactive and heat labile chemicals.
3- Living organism- where reconstituted cells after drying must be able to grow and multiply to produce new progency.
4- miscellaneous- Flood damaged books, museum, artifacts etc.
LYOPHILIZATION PRINCIPLE-
The basic principle in lyophilization is sublimation, in which the conversion from a solid directly into a gas occur. Just like evaporation, sublimation occurs when a molecule gains enough energy to break free from the molecules around it. Water will sublime from a solid (ice) to a gas (vapour) when the molecules have enough energy to break free but the conditions aren't right for a liquid to form. There are two major factors that determine what phase (solid, liquid or gas) a substance will take: heat and atmospheric pressure. Without these conditions, that phase of the substance can't exist.
We can observe from fig. 4 that, water can take a liquid form at sea level (where pressure is equal to 1 atm) if the temperature is in between the sea level freezing point (32 degrees Fahrenheit or 0 degrees Celsius) and the sea level boiling point (212oF or 100oC). But if we increase the temperature above 32oF while keeping the atmospheric pressure below 0.06 atmospheres (ATM), the water is warm enough to thaw, but there isn't enough pressure for a liquid to form. It becomes a gas.13
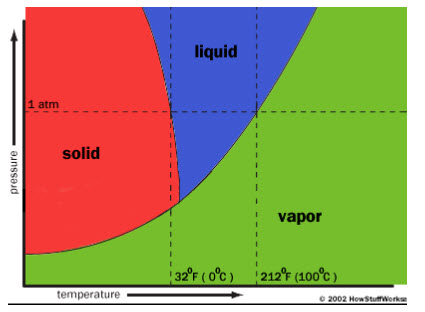
fig. 4 shows the necessary pressure and temperature values of different phases of water.
Tom Harris et al.,“ How stuff Works How freeze drying works”
FREEZ DRYING PROCESS-
A typical freeze-drying process consists of three important stages, that is, freezing, primary drying, and secondary drying. In Freezing, most of the solvent, typically water, is separated from the solutes to form ice. The freezing stage typically takes several hours to finish. Primary drying, or ice sublimation, begins whenever the chamber pressure is reduced and the shelf temperature is raised to supply the heat removed by ice sublimation. During primary drying, the chamber pressure is well below the vapour pressure of ice, and ice is transferred from the product to the condenser by sublimation and crystallization onto the cold coils/plates (<−50°C) in the condenser. Secondary drying is the stage where water is desorbed from the freeze concentrate, usually at elevated temperature and low pressure.13
1. Freezing-
Freezing is a critical step in freeze drying process since the micro structure formed during freezing determines both the quality of the final product and its processing characteristics, such as the rate of primary drying and secondary drying. The product must be frozen to low temperature to a point where it is completely solidified. Freezing the product decreases chemical activity by decreasing molecular movement.
In general, freezing is defined as the process of ice crystallization from super cooled water. The freezing process first involves the cooling of the solution until ice nucleation occurs. Ice crystals begin to grow at a certain rate, resulting in freeze concentration of the solution, a process that can result in either crystalline or amorphous solids or in mixtures 14. Freezing an aqueous pharmaceutical formulation can be conducted at a temperature at below -35oc.
The phenomena that take place in freezing step are-
1.1. Super-cooling
The retention of the liquid state below the equilibrium freezing point of the solution is termed as “supercooling”. It always occurs during freezing often in the range of 10-15°C or more.15
Super-cooling is of two types,
1.1.1. Global super-cooling
It is the process in which the entire liquid volume exhibits a similar level of super cooling
1.1.2. Local super-cooling
In this method, only a small volume of the liquid is super cooled. Super-cooling is a non equilibrium, meta-stable state, which is similar to an activation energy necessary for the nucleation process
1.2. Ice-nucleation
Due to density fluctuations from Brownian motion in the super-cooled liquid water, water molecules form clusters with relatively long-living hydrogen bonds with similar molecular arrangements as in ice crystals.16
Nucleation is of two types
1.2.1. Homogeneous nucleation
The limiting nucleation temperature of water is referred to as the “homogeneous nucleation temperature” that appears to be at about -40°C. At this temperature, pure water sample will contain at least one spontaneously formed active water nucleus, capable of initiating ice crystal growth.
1.2.2. Heterogeneous nucleation
In heterogeneous nucleation ice-like clusters are formed via adsorption of layers of water on “foreign impurities”.
1.3 Ice crystal growth
Once the critical mass of nuclei is reached, ice crystallization occurs rapidly in the entire system which leads to the formation of stable ice crystals. Once stable ice crystals are formed, their growth proceeds by the addition of molecules to the interface. As crystallization
begins, the product temperature rises rapidly to near the equilibrium freezing point. After the initial ice network has formed, additional heat is removed from the solution by further cooling and the remaining water freezes when the previously formed ice crystals grow. The number of ice nuclei formed, the rate of ice growth and the ice crystals size depend on the degree of super-cooling.17
2. Primary Drying
It is characterized by receding boundary layer of ice in the vial. This step traditionally is carried out at chamber pressures of 40-400 Torr and shelf temperatures ranging from -30°C to -10° C. In this phase the chamber pressure is reduced up to 0.01 to 0.1mbar by introducing vacuum in to the product chamber. Heat is applied to the product to cause the frozen mobile water to sublime. The water vapour is collected on the surface of a condenser. The condenser must have sufficient surface area and cooling capacity to hold all the sublimed water from batch at a temperature lower than the product temperature. If the temperature of the ice on the condenser is warmer than the product, water vapour will tend to move towards the product, and the drying will stop. Sublimation front and the partial pressure of water vapour in the freeze dry chamber. Since vapour pressure is related to temperature, it is necessary that the product temperature is warmer than the condenser temperature. The molecules of water move from higher vapour pressure region in the chamber towards the lower vapor pressure region in the condenser. It is extremely important that the temperature at which a product is freeze dried is balanced between the temperature that maintains the frozen integrity of the product and the temperature that maximizes the vapour pressure of the product. This balance is key to optimum drying. The condenser is kept at a low temperature, generally around –60ºC. The temperature of the product should be kept as close to the glass transition temperature as possible for maximum efficiency in drying.18
3. Secondary drying
After primary freeze-drying is complete and all ice has sublimed, bound moisture is still present in the product. The product appears dry, but the residual moisture content may be as high as 7 -8%. Therefore, continued drying is necessary at warmer temperature to reduce the
residual moisture content to optimum values. This process is called ‘Isothermal Desorption’ as the bound water is desorbed from the product. This step is accomplished by raising the shelf temperature to higher than ambient conditions. The shelf temperature can be raised to 15-300C, for allowing the water molecules to desorbs under vacuum. The shelf temperature should not be raised above the product temperature; otherwise degradation of the product occurs. The product may appear to be dry at the end of the primary drying stage but, the moisture content may still be 7-8% weight. Secondary drying continues until the desired moisture content of the product is achieved. The moisture content should be less than or equal to 2% of the product weight. The product should not be over dried, and should not have final moisture content below 1.5 %weight in order to preserve the cake structure. Some chemotherapeutics and antibiotics can have moisture contents as low as 0.1 weight%. Secondary drying parameters are based on the quantity and nature of residual water in the product and the absorption, adsorption and desorption processes. It is also important to know how much heat the product can withstand without degrading and the shelf temperature should not be raised above this temperature.18
FORMULATION OF FREEZ DRYING-
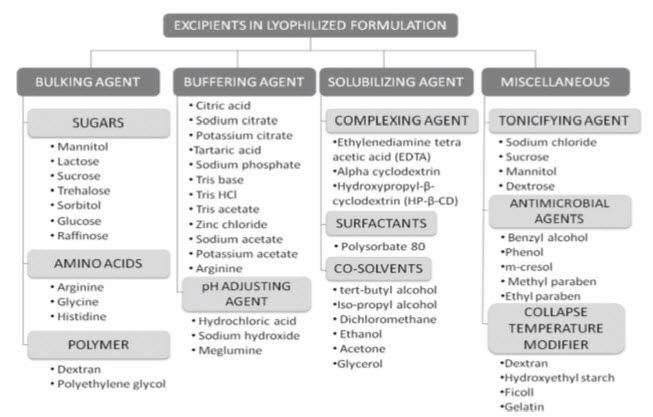
Figure 5 Classification of commonly used excipients used in lyophilization of small molecules. 19
Buffers : Buffers are required in pharmaceutical formulations to stabilize pH. E.g. Phosphate buffers, especially sodium phosphate, Tris, citrate, and histidine buffers.20
Bulking Agents: The purpose of the bulking agent is to provide bulk to the formulation. This is important in cases in which very low concentrations of the active ingredient are used. Crystalline bulking agents produce an elegant cake structure with good mechanical properties. However, these materials often are ineffective in stabilizing products such as emulsions, proteins, and liposome’s but may be suitable for small-chemical drugs and some peptides e.g. Mannitol, Sucrose or one of the other disaccharides.20
Stabilizers: In addition to being bulking agents, disaccharides form an amorphous sugar glass and have proven to be most effective in stabilizing products such as liposomes and proteins during lyophilization. Sucrose and trehalose are inert and have been used in stabilizing liposome, protein, and virus formulations. Glucose, lactose, and maltose are reducing sugars and can reduce proteins by means of the Mailard reaction. Two hypotheses have been postulated to explain the stabilizing effects of the disaccharides.20
1. The water replacement hypothesis: Disaccharides have been found to interact with these products by hydrogen bonding similarly to the replaced water.
2. The vitrification hypothesis: Disaccharides form sugar glasses of extremely high viscosity. The drug and water molecules are immobilized in the viscous glass, leading to extremely high activation energies required for any reactions to occur.
Tonicity Adjusters: The need for such a formulation may be dictated by either the stability requirements of the bulk solution or those for the route of administration. Excipients such as mannitol, sucrose, glycine, glycerol, and sodium chloride are good tonicity adjusters.20
VARIOUS EQUIPMENTS FOR LYOPHILIZATION-
Production freeze-dryer: Tray freeze-dryers are more sophisticated and are used to dry a variety of materials. A tray freeze-dryer is used to produce the driest product for long-term storage. A tray freeze-dryer allows the product to be frozen in place and performs both primary (unbound water removal) and secondary (bound water removal) freeze-drying, thus producing the driest possible end-product21

(fig. 6).
FIG. 6: PRODUCTION FREEZE DRYER Tray freeze-dryers can dry product in bulk or in vials. When drying in vials, the freeze-dryer is supplied with a stoppering mechanism that allows a stopper to be pressed into place, sealing the vial before it is exposed to the atmosphere. This is used for long-term storage, such as vaccines.
Bench top manifold Freeze-Dryer: There are essentially three categories of freeze-dryers: rotary evaporators, manifold freeze-dryers, and tray freeze-dryers. Rotary freeze-dryersareusually used with liquid products, such as pharmaceutical solutions and tissue extracts.

FIG 7: BENCH TOP MANIFOLD FREEZE DRYER21
Proteins and Peptides: 22,23,24
1. Protein G
2. White fish protein
3. LDH
4. G-6 PDH
5. Beta galactosidase catalase
Vaccines: 22,23,24
1. Small pox vaccine
2. Varicella vaccine
3. BCG vaccine
4. MMR vaccine
Drugs:22,23,24
1. Loperamide
2. Domperidone malate
3. Fluorescein
4. Calcitonin
CONCLUSION-
Lyophilization is a commonly used technique for formulation development of small molecules which are unstable in aqueous medium and/or are thermolabile in nature.Lyophilization of drug alone, however, presents certain formulation development challenges, which may be overcome by incorporation of excipients (e.g. bulking agents, buffering agents, tonicifying agent, wetting agent and cosolvents, preservatives and collapse temperature modifiers) in the formulation. A need-based approach should be employed for proper selection of excipients in the formulation for lyophilization, so as to keep the formulation simple for easier processing, while simultaneously maintaining an optimal functionality.
REFRENCE:
1. Louis Rey,” Sage of freeze drying” CH-1010. 5-8.2.www.pharmatech.com
3. Nail SL and Gatin LA: Freeze drying: principles and practice, in Avis KE Lieberman HA and Lechman L (eds): Pharmaceutical Dosage Forms:Parenteral Medications, Marcel Dekker, New York. 1993.
4. Gatin LA, Auffret T, Shalaev EY, Speaker SM and Teagarden DL: Freeze Drying Concepts: The Basics, in McNally EJ and Hastedt JE (eds): Protein Formulation and Delivery, Informa Healthcare, New York, NY, Vol. 175, 2008.
5. Pikal MJ: Freeze drying, in Swarbrick J (ed): Encyclopedia of Pharmaceutical Technology, Informa Healthcare, USA, Vol. 3, 2007.
6. Greiff D: Development of cycles for lyophilization. Dev Biol Stand, 74: 85- 92, 1992.
7. Carpenter JF, Pikal MJ, Chang BS and Randolph TW: Rational design of stable Lyophilized protein formulations: some practical advice. Pharm Res, 14: 969- 975, 1997.
8. Craig DQM, Royall PG, Kett VL and Hopton ML: The relevance of the amorphous state to pharmaceutical dosage forms: glassy drugs and freeze dried systems. Int J Pharm, 179: 179- 207, 1999.
9. Yoshioka S, Aso Y and Kojima S: The effect of excipients on the molecular mobility of lyopihilized formulations, as measured by glass transition temperature and NMR relaxation-based critical mobility temperature. Pharm Res,16: 135-140, 1999.
10. Wang W: Lyophilization and development of solid proteinpharmaceuticals. Int J Pharm, 203: 1-60, 2000.
11.International Pharmaceutical Excipients Council of the Americas. http://ipecamericas.org - Question 1
12.Nail SL, Jiang S, Chongprasert S, Knopp SA. Fundamentals of freeze- drying. Pharm Biotechnol. 2002, 14, 281-360.
13.M. J. Pikal. Lyophilization. In J. Swarbrick and J. Boylan (eds.),Encyclopedia of Pharmaceutical Technology, Marcel Dekker, New York, 2002,
14.Franks F and Auffret T. Freeze-Drying of Pharmaceuticals and Biopharmaceuticals, RSC Publishing, Cambridge, UK, 2007.
15. Searles JA. Freezing and Annealing Phenomena in Lyophilization, in: L.Rey J.C. May (Eds.) Freeze- Drying/Lyophilzation of Pharmaceutical and Biological Products, Marcel Dekker, Inc. USA, New York, 2004
16. Matsumoto M, Saito S, Ohmine I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature. 2002
17. Rambhatla S, Ramot R, Bhugra C, Pikal M. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of supercooling, AAPS Pharm Sci. Tech. 2004, 5, 54-62
18. Gannu Praveen Kumar*, Nooka Prashanth, Bairi Chaitanya Kumari.Review paper on Fundamentals and Applications of Lyophilization IN Journal of Advanced Pharmaceutical Research. 2011.
19. Bahetia A, Kumarb L, Bansal AK. Excipients used in lyophilization of small molecules. IPEC-Americas Inc J. Excip. Food Chem. 2010.
20. Frank Kofi Bedu-Addo, PhD et al.“Understanding Lyophilization Formulation Development” Biopharmaceuticals. 10-18.
21. wikipedia.com
22. The Theory and Practice of Industrial Pharmacy By LEON LACHMAN, HERBERT A. LIEBERMAN, JOSEPH L. KANIG, Third Edition, Varghese Publishing House, 672
23. Pharmaceutical Dosage forms Parenteral Medications Second edition, Eddited by Kenneth E.Avis, Herbert A. LIBBERMAN,& Leon lechman Volume-1, 217-228.
24. The science and Practice of Pharmacy 21st edition volume 1 by REMINGTON,. 828-831
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









