{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Himani Devliyal*, S.S Agarwal
Department of Pharmacology,
Delhi Institute of Pharmaceutical Sciences and Research, Delhi University, New Delhi
himanidevliyal@gmail.com
ABSTRACT
Centchroman – A Non-steroidal, post-coital anti-implantation drug which is generally popular as SAHELI is one of the best agents available. The fundamental aim of this study was to formulate such an Intra-uterine System of Centchroman which should be safe and acceptable. This new drug delivery system surpasses the first pass metabolism, thus increasing the bioavailability and reducing the drug load. This formulation delivered the drug upto 21 days. Polylactic-co-glycolic acid served as the best fit polymer for delivering the drug. The best optimized formulation was selected and subjected to stability studies. The effect of the foreign bodies was also observed on the fertility, mating behaviour and estrous cycle of the rats along with anti-implantation activity of the IUS. This formulation was tested on Wistar albino rats and is proved to be safe at the optimized dose level. The formulation exhibited pre-implantation loss of 100% and post-implantation loss of 0% in Wistar albino rats at a particular dose when delivered on 1st day and 4th day post-coital. Experimental part on animals showed that any foreign body, if put inside the uterus would not affect the fertility unless the size is more than 6mm. Results showed that 3mm objects had no effect on fertility. The foreign bodies and the IUS showed neither any alteration in the estrous cycle nor on the mating behavior of the animals.
INTRODUCTION
In the recent years, considerable attention has been focused on enhancing the drug absorption and efficacy by redesigning the delivery process rather than the drug molecules or chemical entities. This has led to the development of Novel Drug Delivery System10. There are several advantages of Intra-uterine delivery as opposed to oral delivery, for e.g. drug avoid the hostile environment of the GIT (the action of pH and enzyme) and avoid first pass metabolism (the drug is directly been delivered to the uterine cavity and thus to systemic circulation) . In addition, drug delivery by the IUS can show sustained plasma profile over long period of time. This could minimize the risk of fluctuation of drug plasma level. Other attractive feature is that it can be inserted once and can be terminated when the lady wants to conceive. The contraceptive options present today are oral pills, IUDs, condoms, vaginal creams etc. Amongst them the most acceptable method of choice is either the oral pills or intrauterine devices8; still there lies a large unmet need for contraception.
Thus our fundamental aim for carrying out this research work was to formulate such a novel delivery system which should be safe and acceptable4. For that purpose we have chosen centchroman9 which is a Non-steroidal post- coital contraceptive agent of established anti-implantation activity2. This drug candidate served a great fit to our set criteria for research work. This property has boosted its efficacy with minimizing side effects. Using centchroman in the IUS form is a novel thought and delivering the same for 21 days was an add-on feature to the formulation. For the better safety and use the drug was dispersed in the polymer PLGA uniformly. PLGA is a biodegradable polymer, therefore the device need not to be removed from the body when not required further, thus increasing patient compliance. Moreover the polymer was found to be highly compatible with the drug. This formulation is tested on Wistar albino rats and is proved to be safe at the optimized dose level. The efficacy and safety of the IUS was compared with the oral drug5. During the Pharmacodynamic evaluation of the IUS the effect of presence of foreign bodies was also figured out through the same1. The present study was undertaken to evaluate the effect of IUS on the fertility. The study was done to determine the effect of time, site and length of foreign bodies on fertility. As foreign bodies, Nylon thread, surgical suture and leaf petiole were selected. These were placed in the lower segment of the right uterine horn. The distance of the vagina from the cervix was optimized previously. The piston was allowed to push the patch upto the lower segment of the horn. The length of the foreign agent taken ranged from 3mm to 6mm.
MATERIALS AND METHODS
Animals: Wistar albino female rats having weight between 150-200 g were used in the present study. Our Protocol No.7 (IAEC) of the year 2006-07, was approved by “Institutional animal ethics committee” (IAEC), as per the norms of CPCSEA (Committee for the Purpose of Control & Supervision of Experimentation on Animals). Healthy albino rats were obtained from animal house, of DIPSAR, Pushp Vihar, New Delhi (Registration no. 215/CPCSEA 1ST June 2000). All the animals were kept under identical conditions of food, water, and temperature at DIPSAR animal house.
Materials: The drug sample of centchroman was obtained as gift sample from Hindustan Latex Ltd. Batch No. CENT F002, Manufacturing date Oct /2006. UV spectroscopy, FTIR and DSC authenticated the drug sample.PLGA used was provided by Supreme combine Bombay. Animal ultrasound machine was used during the study for evaluating the implants of the animals.
Methods: Centchroman sample was authenticated byUV, FTIR and DSC. Standard curve of Centchroman was prepared and the absorbance was obtained at 278nm. Matrix type IUDDS was prepared using solvent casting technique in which the drug was dispersed uniformly in the polymer that is PLGA3. 2% propylene glycol was added to the formulation as a plasticizer. The formed film containing the dispersed drug was stripped off and kept in oven at 400C for 3-4 hrs. Total of five formulations were prepared, out of which one was optimized which showed the highest release rate among all. The formulation was tested for physical parameters like thickness, weight, area volume and uniformity assay. The dissolution studies were carried out in 230 ml of phosphate buffer pH 7.2 at a temperature of 37ºC at a constant speed of 50 rpm. The developed film was subjected to accelerated stability testing according to the guidelines prescribed by International Conference on Harmonization (ICH). The films were kept at 40ºC temperature and 75% relative humidity. The films were packaged with butter paper and aluminum foil (Blister pack). These packs were then put into a resealable plastic bag and subjected to 40º C at 75 % RH and 25º C at 65 % RH in an incubator. The films were analysed for Drug release profile and Polymer-drug interaction/Polymer-polymer interaction (FTIR & DSC)
Further in-vivo animal study was conducted which accounted for the efficacy and safety of the system formulated. In addition a partner study was also conducted to determine the effect of time, site and length of foreign bodies on fertility. As foreign bodies, Nylon thread, surgical suture and leaf petiole were taken. These were placed in the lower segment of the right uterine horn. The patch was delivered through the vagina of the animal .For that a polyethylene tough tube of diameter 2mm was used inside which the patch was rolled in. The patch was then pushed through another tube which acted as a piston. The distance of the vagina from the cervix was optimized previously which is about 2.8 ± 0.5 cm .The piston was allowed to push the patch upto the lower segment of the horn. The position of the patch was confirmed by looking inside the uterus of the few animals slaughtered after insertion. The length of the foreign bodies varied from 3mm to 6mm also.6, 7
RESULTS:
Mean of readings of the same specimen was taken as actual value. Data were presented as mean±SEM. One-way ANOVA followed by Tukey test was used to compare mean values of the groups.
The dissolution of the optimized formulation showed a release of 5.12,19.31, 45.66, 80.96, and 98.66 % ofthe drug in 1, 18, 108, 263 and 479 hr respectively. The regression coefficient value of Higuchi’s plots was calculated to be 0.9944.Thus the formulation was following higuchi’s release pattern giving it a confirmation of sustain release.(fig-1)
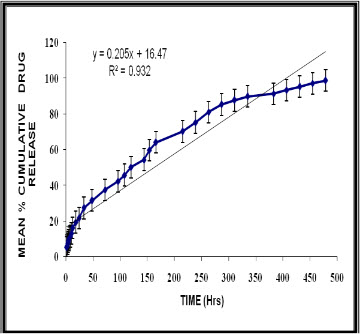
Figure 1 Depicts mean % cumulative release of the optimised formulation
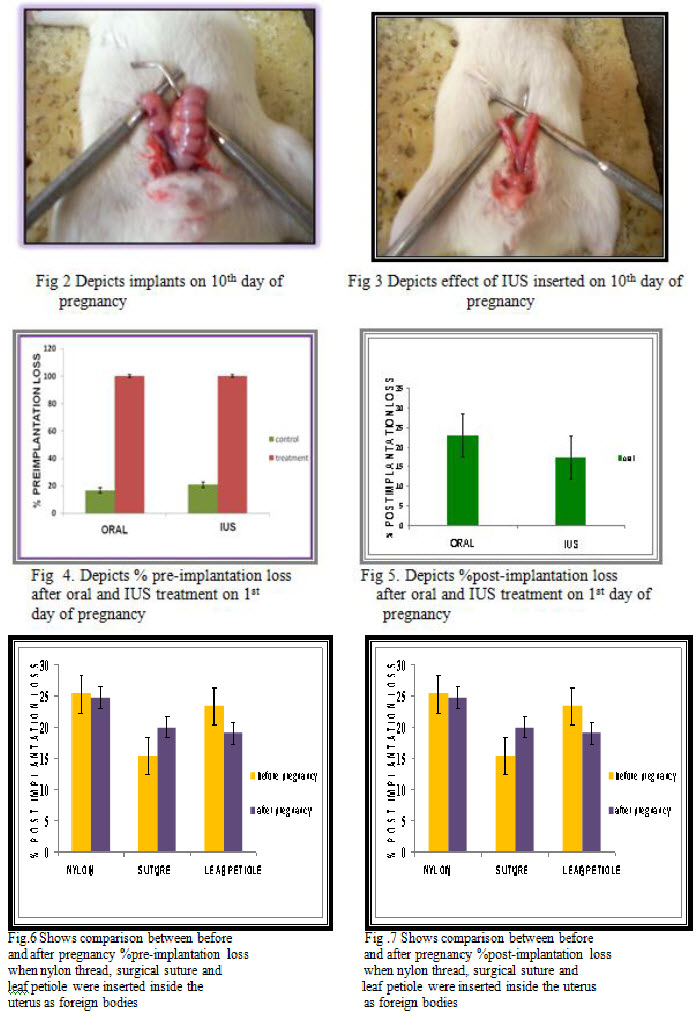
For carrying out animal studies 3 groups were made which were classified into orally treated, Foreign body inserted and IUD inserted. Each group comprised of 6 animals. Oral control groups were administered with 2% w/v gum acacia solution while uterine controlled groups were inserted with blank polymeric films. The test group was inserted with the drug loaded patch. The dose volume employed for the oral dosing was 3.1mg/kg .Dosing was done taking into consideration the individual weight of the animals .The drug solution was introduced into the lower part of the esophagus using an oral feeding catheter. The effect of foreign bodies on pregnancy was also seen by inserting various agents like nylon thread, leaf petiole and suture
The uterine control group of animals, in which blank patch was inserted before pregnancy and was kept for mating the same day showed % pre-implantation loss of 23.06% + 4.97 and % post-implantation loss of 20.48% + 5.71 while the uterine control group of animals, in which drug loaded patch was inserted before pregnancy and was kept for matingthe same day, showed % pre-implantation loss of 0 % and % post-implantation loss of 0%.
The Analysis of Variance calculation for the in vivo study of Centchroman resulted F(5,66) value of 0.035 and was found to be less than that of tabulated value of F (0.05) (P >0.05) which was 0.06 therefore, statistical significance in the resultsof intrauterine administered Centchroman was observed. Tukey test between the drug loaded patch of centchroman and blank patch resulted in a P-value less than 0.05 and was considered very significant i.e. there was very significant difference between the two groups.
DISCUSSION:
The main aim of this research work was to formulate and evaluate the matrix type Intrauterine drug delivery system for Centcroman using a biodegradable polymer PLGA. The polymer offers several advantages of being biocompatible, biodegradable, non-toxic and cost effectiveness along with displaying suitable controlled release characteristic of the controlled drug delivery system. The pharmacodynamic effects of the optimized patch shows an anti-fertility activity of 100%.The effect of foreign bodies on the fertility depends on many factors. The most vital amongst them is the size of the foreign body and its location inside the uterus. It has been seen during the study that a foreign agent of 3mm does not produce any antifertility effect but of 6mm can probably affect the pregnancy. Similarly the position of that body inside the uterus affects the fertility accordingly.
There was no effect on the estrous cycle and mating behaviour of animals when foreign agents were inserted. The same treatment i.e. control and test was given on the 4th day (day of implantation) just to ensure that the IUS is going to affect the implantation site leading to abortion.
CONCLUSION: Considering the results of the present work, it can be concluded that the formulated IUS containing half the oral dose of centchroman has the advantages of lesser dose, less frequent administration and better user compliance. It has been estimated to be of great success if used commercially, as it reduces user compliance too.
ACKNOWLEDGEMENT:
We are grateful to the participants for taking part in the study. Hindustan Liver Ltd. for providing the drug sample and Supreme combine for providing the polymer. We are also thankful to the DIPSAR animal house for providing healthy animals.
REFERENCES
1. Alan J, Margolis M.D and Doyle L.L. Intrauterine Foreign body. Inhibition of Decidual function response in rats. Fertility and sterility 1964;15:607-16
2. CDRI, Centchroman: contraceptive.drugs Fut 1977; 11:441-5.
3. Chasin M,and Langer R.Biodegradable Polymers as drug Delivery Systems;1990:1-10
4. Chein YW. Novel Drug Delivery Systems.1982.
5. Dickmann Z.Implantations in the rat: progesterone priming requirements.J Endocrinol 1972;53: 327-32
6. Doyle L.L and Margolis M.D. Intrauterine Foreign body. Effect on pregnancy in the rat. Science 1963 : 833
7. Doyle L.L, Alan J, Margolis M.D .Intrauterine Foreign body. Effect on reproductive process in rat. Fertility and sterility.15;1964:597-605
8. Hugh J, Davis M.D. Intrauterine contraceptive devices: Present status and future prospects. Am.J Obstert.Gynecol;1972,114: 134 -51
9. Indian Pharmacopoeia. Ormeloxifine hydrochloride,1996;1:539-540
10. Robinson JR, Park K. Controlled drug Delivery: challenges and strategies. American Chemical Society 1997; 3(1): 63-72.
REFERENCE ID: PHARMATUTOR-ART-2167
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 5 Received On: 20/03/2014; Accepted On: 27/03/2014; Published On: 01/05/2014 How to cite this article: H Devliyal, SS Agarwal; Formulation, Development and Evaluation of Anti-Implantation Activity of Intrauterine System of Centchroman (Saheli) using Wistar Albino Rats; PharmaTutor; 2014; 2(5); 128-132 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









