 About Author: Rajyalakshmi Dhulipati (B.Pharm), Janardhanreddy Ramareddy P. (M.Pharm), G. Rajalakshmi (M.Pharm, Lecturer), Dr. N. Damodharan (M.Pharm, Ph.D, Professor & Head of the Department)
About Author: Rajyalakshmi Dhulipati (B.Pharm), Janardhanreddy Ramareddy P. (M.Pharm), G. Rajalakshmi (M.Pharm, Lecturer), Dr. N. Damodharan (M.Pharm, Ph.D, Professor & Head of the Department)
Department of Pharmaceutics,
SRM College of Pharmacy, SRM University,
Kattankulathur-603203, Kancheepuram Dist., Tamil Nadu.
Reference ID: PHARMATUTOR-ART-1055
Abstract
The main aim of the study is to formulate and evaluate floating microspheres of Metformin Hydrochloride. Gastro retentive floating microspheres have emerged as an efficient means of enhancing the bioavailability and controlled delivery of many drugs. Floating microspheres of microspheres are formulated in order to achieve an extended retention time in upper G.I.T. which results in enhanced absorption and thereby improving the bioavailability. Floating microspheres were prepared by Non aqueous solvent evaporation method by using Eudragit RS 100 and Eudragit RL 100. The pure drug, the polymers and the physical mixtures were evaluated for FTIR. The prepared Metformin hydrochloride microspheres were evaluated for surface topography, angle of repose, bulk density, tapped density, carr’s index, hausner’s ratio, yield of microspheres, particle size analysis, drug entrapment efficiency, invitro floating ability, invitro drug release and kinetic studies. Results showed that there were no drug incompatibilities and there was good floating ability. SEM revealed the size and surface morphology. The Preformulation parameters were found to be satisfactory. Drug entrapment efficiency was found to be 70-135% and yield was found to be 70-95%. The drug release profiles showed that microspheres prepared with Eudragit RS 100 showed less release when compared to Eudragit RL 100. When Eudragit RL 100 was used in combination with Eudragit RS 100, the release was found to be faster when compared to Eudragit RS 100 alone. The release data obtained were fitted to Higuchi and Korsemeyer-Peppas model to indicate that the mechanism of the drug release was non fickian type of diffusion.
[adsense:336x280:8701650588]
Introduction
Oral ingestion has been the most common and conveniently employed route of drug delivery. Floating microspheres are gastro retentive drug delivery system based on non effervescent approach. They are low density systems that have sufficient buoyancy to float over gastric contents and remain in stomach for prolonged period of time. As the system floats over gastric contents, the drug is released slowly at desired rate resulting in increased gastric retention with reduced fluctuations in plasma drug concentration. When microspheres come in contact with gastric fluid the gel formers, polysaccharides and polymers hydrate to form a colloidal gel barrier that controls the rate of fluid penetration into the device and consequent drug release. As the exterior surface of the dosage form dissolves, the gel layer is maintained by the hydration of the adjacent hydro colloidal layer. The air trapped by swollen polymer lowers the density and confers buoyancy to the microspheres. In present study, floating microspheres of Metformin hydrochloride are formulated in order to achieve an extended retention in upper G.I.T which results in enhanced absorption and there by improved bioavailability.(1)
MATERIALS AND METHODS:
Metformin hydrochloride (Emcure pharmaceuticals, pune), Eudragit RS 100 and Eudragit RL 100 (Rohm Pharma, Germany), magnesium stearate, pet. Ether, acetone and liquid paraffin (LAB chemicals, Chennai).
MATERIALS:
Materials used and their suppliers
|
Materials |
Suppliers |
|
Metformin Hydrochloride |
Emcure Pharmaceuticals, Pune. |
|
Eudragit RS100 |
Rohm Pharma, GmbH, Darmstadt, Germany |
|
Eudragit RL100 |
Rohm Pharma, GmbH, Darmstadt, Germany |
|
Magnesium Stearate |
LAB Chemicals, Chennai |
|
Acetone |
LAB Chemicals, Chennai |
|
Petroleum ether (40-60°) |
LAB Chemicals, Chennai |
|
Liquid Paraffin |
LAB Chemicals, Chennai |
EQUIPMENTS:
List of equipments used and their manufacturer
|
Equipments |
Manufacturer |
|
UV-VIS spectrophotometer |
Schimadzu |
|
FTIR spectrophotometer |
Perkim Elimer |
|
Dissolution apparatus (USP XX II) |
Disso 2000 |
|
Scanning Electron Microscopy |
Leica (S430), UK. |
|
Electronic Balance |
Cyber Lab |
|
Magnetic Stirrer |
REMI Equipment (P) Ltd |
|
Hot air oven |
Sansel |
|
pH meter |
Susima Technologies (P) Ltd |
METHOD OF PREPARATION:
Floating microspheres containing Metformin hydrochloride by non aqueous solvent evaporation method. The drug and magnesium stearate were mixed thoroughly in polymer solution at various ratios. The slurry was slowly introduced into liquid paraffin stirred at 1200 rpm for 2 hrs and solvent was allowed to evaporate completely and filtered. Then they are washed repeatedly with petroleum ether until free from oil. Finally they are dried for an hour at room temperature and kept in dessicator.(8)
EVALUATION PARAMETERS(5,6,7,12,14):
IR spectra:
Spectra of pure drug, polymers and physical mixtures were obtained in KBr pellets at moderate scanning speed between 4000-200 cm-1.
Surface topography:
The samples were mounted on gold palladium alloy of 120 A° thickness was coated on the sample using sputter coating unit in argon ambient of 8-10 with plasma voltage about 2 KV and discharge current about 20 MA. The photomicrographs were recorded at 500x, 1000x.
[adsense:336x280:8701650588]
Angle of repose:
It was done by fixing the dry funnel to burette stand at a particular height. A graph paper was placed below to it and quantity of microspheres was allowed to flow slowly. A circumference of heap was carefully drawn and midpoint was located to calculated average radius.
Tan ? = h/r
Bulk Density:
Bulk density of a compound various substantially with the method of crystallization, milling or formulation. Bulk density is determined by pouring presieved microspheres into a graduated cylinder via a large funnel and measure the volume and weight.
Bulk density= weight/ bulk volume
Tapped Density:
Tapped density is determined by placing a graduated cylinder containing a known mass of microspheres and mechanical tapper apparatus, which is operated for a fixed number of taps until the volume has reached a minimum volume. Using the weight of the drug in the cylinder and this minimum volume, the taped density may be computed.
Tapped density= weight/tapped volume
Carr’s index:
It can be measured of potential strength that powder could build up in its arc in hopper and also the case with an arch could be broken.
Carr’s index= Tapped density- BulK density/ Tapped density
Hausner’s ratio:
It is an indirect index of case of measuring the powder flow. It is calculated by the following formula.
Hausner’s ratio = Tapped density/Bulk density
Particle size analysis:
Size distribution was determined by sieving the micro particles using mesh no # 36 as well as by optical microscopy counting at least 100 microspheres.
Drug entrapment efficiency:
It was performed by accurately weighing 50mg of microbeads and suspending in 100 ml of pH 7.4 and it was kept on a side for 24 hours. Then, it was stirred for 15 mins and filtered. After suitable dilution, Metformin hydrochloride content in the filtrate was analyzed spectrophotometrically at 233 nm using U.V.Spectrophotometer.
% entrapment efficiency = (actual drug content/theoretical drug content)*100
Invitro floating ability:
100 mg of microspheres were placed in 0.1 N Hcl containing 0.02% tween 20 and stirred at 100 rpm. The layer of buoynant microspheres was filtered at 1, 2, 4 and 6 hrs. The collected microspheres were dried in dessicator over night.
%floating microspheres = (final wt/initial wt) × 100
Table 1 Composition of Floating Microsphere
|
INGREDIENTS |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
|
Metformin Hcl |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Eudragit RS100 |
1 |
2 |
3 |
- |
- |
- |
1 |
|
EudragitRL100 |
- |
- |
- |
1 |
2 |
3 |
1 |
|
Mg. Stearate |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
|
Acetone |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Liquid paraffin |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
|
Tot. Wt |
2.1 |
3.1 |
4.1 |
2.1 |
3.1 |
4.1 |
3.1 |
Invitro drug release:
Drug dissolution studies were carried out in 0.1 N Hcl in USP type II apparatus at 37±0.5 ºC. The samples were collected from the release medium at regular intervals. After each sample collection, the same amount of fresh release medium at the same temperature was added to the release medium to maintain sink condition. The drug concentration of each sample was determined spectrophotometrically at 233 nm.
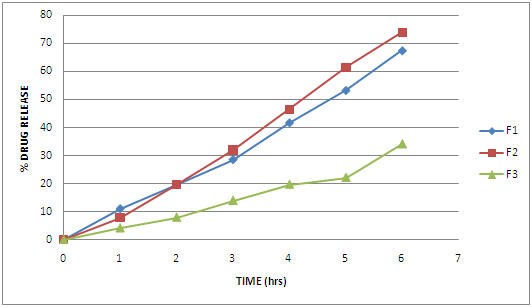
Fig 1 Drug Release From F1, F2 and F3
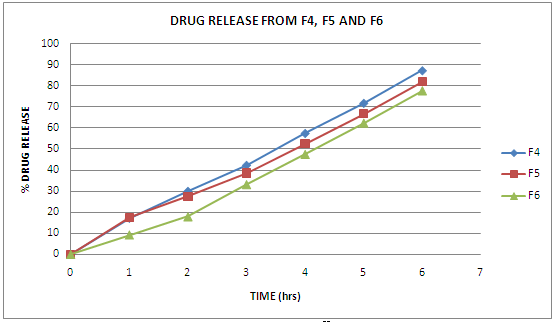
Fig 2 Drug Release From F4, F5 and F6
Kinetics study:
In order to understand the mechanism and kinetics of drug release, the drug release data of the in-vitro dissolution study was analyzed with various kinetic equations like zero-order (% release v/s time), first order ( Log % retained v/s time) and korsmeyer-peppas equation. Coefficient of correlation (r) values were calculated for the linear curves obtained by regression analysis of the above plots.
RESULTS AND DISCUSSION:
The floating microspheres of Metformin hydrochloride was prepared and evaluated. FTIR showed that drug was compatible with excipients. SEM photographs reveal that they are spherical, non porous, uniform with smooth surface. The angle of repose was found to be in the range 20-30° and shows good flow property. The bulk density and tapped density was found to be in the range of 0.350-0.525 g/cm3 and 0.450-0.600 g/cm3 respectively. The carr’s index was found to be in the range 10-20. The hausner’s ratio was found to be in the range 1.11-1.40. Average particle size for prepared microspheres found to be in the range of 200-300 μm. The drug content was uniform and reproducible in each batch. The entrapment efficiency of the drug depends on solubility of the drug in the solvent. An increase in the polymer concentration in fixed volume of organic solvent resulted in an increase in encapsulation efficiency. The entrapment efficiency was found to be 70-135%. The prepared microspheres showed good percentage yield and found to be 70-95%. Floating ability was found to be in the range of 45.09-91.47%. The microspheres prepared with Eudragit RS100 showed considerable sustained release characteristics. The release rate from the formulation 1, 2, 3 upto 6 hrs was found to be 73.88%, 67.45%, 34.19%. Microspheres prepared with Eudragit RS100 decreases with the decrease in polymer concentration. The release was sustained as concentration of polymer increases. This may be due to swelling of polymer and release of drug by diffusion through swelled pores. Microspheres prepared with Eudragit RL100 showed more release as prepared to Eudragit RS100. This may be due to the high content of the ammonium group in Eudragit RL100 which might have facilitated the diffusion of some of the entrapped drug. Drug release from 4, 5, 6 hrs was found to be 87.28%, 81.93%, 77.69% respectively. The release was sustained as concentration of polymer increased.
CONCLUSION:
Gastro-retentive floating microspheres have emerged as an efficient means of enhancing the bioavailability and controlled delivery of many drugs. The control of gastro intestinal transit could be the focus of the next decade and may result in new therapeutic possibilities with substantial benefits for patients.
REFERENCES:
1. D M Brahmankar and Sunil B Jaiswal, Biopharmaceutics and Pharmacokinetics a Trea-tise.
2. Robinson JR and Lee VHL, 1987., ‘Controlled Drug Delivery- Fundamentals and Applications’, 2nd edition, Marcel Dekker, INC, New York, 09.
3. www.wikipedia.org
4. Yie w Chein., ‘Novel drug delivery systems’, 2nd edition, revised and expanded.
5. Anand Kumar Srivastva, Devendra Narayanrao Ridhukar,; Formulation, characterization and Invitro evaluation; Original Research Paper, Acta Pharm., 55(2005)., 277-285.
6. M.K Deepa, M. Karthikeyan; Formulation and invitro evaluation of cefpodoxime proxetil microspheres; Iranian Journal of Pharmaceutical Sciences Spring 2009;
7. V N Deshmukh, D M Sakarkar; Formulation and evaluation of controlled release alginate microspheres using locust bean gum., Research Article., ISSN: 0974-6943.
8. Mostafa Safari, Malihe Shahbazi., Formulation and evaluation of Eudragit L100 microspheres of piroxicam., Nature preceding: doi:10.1038/npre.2008.1544.1:2008.
9. Aulton M E., John C Morten., 2002. Modified release preoral dosage forms, The Science of Dos¬age Form Design, 2nd edition, 290-300
10. British Pharmacopoeia 2009, I
11. Skoog A., Donald M., West F., Holler J., Stanley R., 2004. ‘Fundamentals of Analytical chemistry’, 8th edition
12. Sam T Mathew, S Gayathri Devi., Formulation and evaluation of Ketorolac Tromethamine-loaded albumin microspheres, AAPS PharmSciTech 2007; 8(1)., Article 14.
13. www.aapspharmscitech.org
14. J B Jeevana., Development and evaluation of gelatine microspheres of tramadol hydrochloride, Journal of Young Pharmacist. (https://www.jyoungpharm.in/text.asp?2009/1/1/24/51871)









