{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Mir Sahidul Ali*1, Souvik Mukherjee2, Subhajit Makar2, Gargi Pal3
*1Department of Chemical and Polymer Engineering,
Tripura University, Suryamaninagar, Tripura, India
2Centre for Pharmaceutical Sciences &
Natural Products, School of Basic and Applied Sciences,
Central University of Punjab, Bathinda, Punjab, India
3Department of Pharmaceutical Sciences and Technology,
NSHM Knowledge Campus, Kolkata, India.
ABSTRACT:
Cucurbitacins which are structurally unlike triterpenes found in the members of Cucurbitaceae and several other plant families possess immense pharmacological activity. This diverse group of compounds may substantiate to be important lead molecules for the treatment of cancer. Research focused on these unattended medicinal compound can prove the significance and explore the hidden potential to cure various diseases. These compounds shows their proposed mode of action, probable molecular targets and have future aspects of their use as a medicinal agent. This review is aimed to provide the chemical nature and medicinal property of cucurbitacins.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2646
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 7, Issue 02 Received On: 06/12/2018; Accepted On: 22/01/2019; Published On: 01/02/2019 How to cite this article: Mir, S.A., Mukherjee, S., Makar, S. and Pal, G. 2019. Cucurbitacins a Vibrant Triterpenoid: A Review on its Anticancer Property. PharmaTutor. 7, 2 (Feb. 2019), 43-54. DOI:https://doi.org/10.29161/PT.v7.i2.2019.43 |
INTRODUCTION
Medicinal plants are the nature’s gift to human being to cure disease and make disease free life. Herbal medicine is still the backbone of about 75-80% of the total population (Ali MS et al., 2018). Plant’s secondary metabolites are important sources for scientific and clinical study as well as for new drug development. Many plant synthesized herbal medicines in different formulations used for treatment of various diseases from an ancient time. Traditional or indigenous drugs have special importance. Phytochemicals as secondary metabolites and they can be used in the pharmaceutical industries for producing a potent drug (Nag M et al., 2018). Terpenes are volatile organic compounds which are the major odoriferous constituents of essential oil and e contain two or more 5-carbon isoprene units. Many terpenes contain additional functioning groups, such as carboxyl (COOH), carbonyl (CO), alkoxy (OR) and hydroxyl (OH). It is tested over a long time, and are relatively safe, easily available and affordable (Kupchan, Meshulam, & Sneden, 1978). Many ethno-botanical survey reported that, the local population in different parts of the world including the USA, China, India, Mexico, Morocco, Saudi Arabia, Taiwan, and Trinidad and Tobago depend on medicinal plant and suggested that several medicinal plants have been used as dietary adjuncts for the cure of numerous chronic and severe disease (H Stuppner & Moller, 1993). In India and China, the use of herbal medicines has been commonly used from a long time, as a less expensive way to treat various health problems. The herbal drugs are less toxic with limited side effects compared with synthetic drugs (DINAN et al., 1997). Due to such reasons, traditional and complementary medicines have seen an increase in their popularity for the treatment of different diseases. The WHO has also recommended the initiation of studies to identify and characterize new herbal preparations from traditionally known plants and the development of new effective therapeutic agents, especially in the areas where the lack of safe modern drugs to treat chronic diseases. Cancer is a chronic disease and serious health threats for about seven million people in a year and a big challenge medical science (Makar, Saha, & Singh, 2018). In the ongoing search for more effective and safer drugs, attention is being paid to new and safe medicinal herbs or food components. Although phyto-therapy continues to be used in several countries as in the past, only a few plants have received scientific or medical scrutiny (Kaushik, Aeri, & Mir, 2015). Although most of the herbal plants are safer, still a number of medicinal plants possess some degree of toxicity; therefore it is very important to analyze the traditional therapeutic regimens scientifically and their dosing, toxicity and other health consequences, before proper use in human diseased conditions. Now a days the environment becomes highly polluted with variety of toxic chemical and waste biological active substances such as dyes, polymers, steroids, phytochromes, or the ubiquitous free radicals (Dinan, Harmatha, & Lafont, 2001).Cucurbitacin are terpenoids category of diverse compounds found in the plants of family Cucurbitaceae. These compounds have medicinal and toxic properties. These compounds are also discovered in other plant families like Scrophulariaceae, Cruciferae, Datiscaceae, Primulaceae, Rubiaceae etc. The diversity of Cucurbitacin lies in variety of its side chain derivatives that contribute to their disparate pharmacological actions. The bitter taste of plant species like cucumber have been identified to the presence of Cucurbitacin. The first Cucurbitacin was isolated as a crystalline form substance in 1831 and was named α-elaterin (Gamlath, Gunatilaka, Alvi, & Balasubramaniam, 1988).
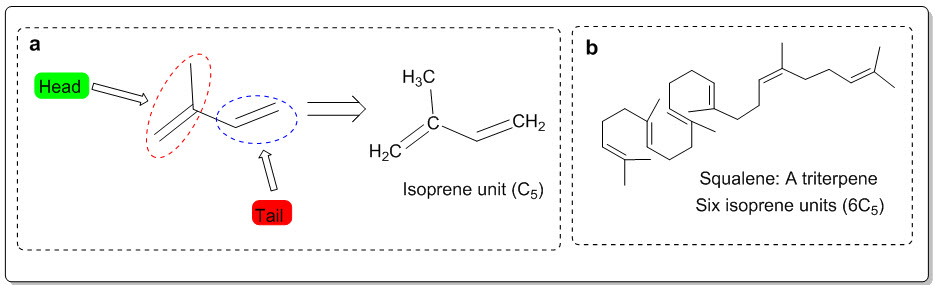
Fig. 1: (a) Isoprene unit and (b) triterpene (Squalene)
2. Occurrence:
Cucurbitacins are found in many cucurbitaceous plants. They are most common in species of the Bryonia, Cucumis, Cucurbita, Luffa, Echinocystis, Lagenaria and Citrullus. The plants of genera Momordica contain a special group of Cucurbitacins called momordicosides. The level of Cucurbitacins varies between tissues. They may be concentrated in fruits and roots of mature plants. In fruits where Cucurbitacins are produced, their highest concentration is achieved on maturity. Seeds generally contain very low concentration of Cucurbitacins. Cucurbitacin producing plants have also been identified outside the cucurbitaceae in the members of Scrophulariaceae, Begoniaceae, Primulaceae, Liliaceae, Tropaeolaceae and Rosaceae. The seeds of certain cruciferous plants, like Iberis species and Lepidium sativum also contain cucurbitacins. It is reported that Cucurbitacins are formed in situ and are not transported to another parts of the plant. The distribution of Cucurbitacins among several families of plant kingdom (Hermann Stuppner, Müller, & Wagner, 1991).
3. Chemistry of Cucurbitacins:
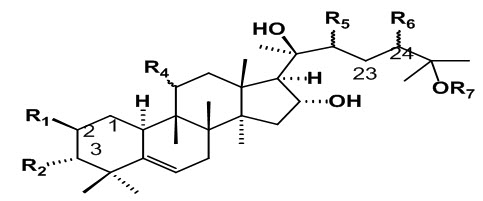
Fig.2: General structure of Cucurbitacin (Gry, 2006)
Cucurbitacins are a group of highly oxygenated triterpenoid molecules found in diverse species of cucurbitaceae family (Zheng, Fu, & Pei, 2007). They are derived from the cucurbitane scaffold. That are widely present intraditional Chinese medicines, possess strong anticancer activity, andare divided into 12 classes from A to T with over 200 derivatives. All cucurbitacins contain a basic 19-(10→9β)–abeo--10α–lanost–5--ene ring skeleton. A common feature among all compounds in the category of Cucurbitacins is the presence of 5, (6)-double bond. The difference of Cucurbitacins from steroidal moiety lies in the fact that in basic structure of Cucurbitacins a methyl group is located at C-9 rather than C-10. Most of the Cucurbitacins are tetracyclic, but some representatives have an additional ring due to formal cyclization between C-16 and C-24 as in cucurbitacins S and T. The Cucurbitacins differ from most of the other tetracyclic triterpenes by being highly unsaturated and contains numerous keto, hydroxyl, and acetoxy groups. Certain Cucurbitacins have been discovered in the form of glycosides and some of them lack C-11 carbonyl function. Chemically, Cucurbitacins are ranked according to presence of various functional groups on rings A and C, diversity in side chain and stereo chemical considerations. The structural composition of following Cucurbitacins are known and have been designated by the letters: A, B, C, D, E, F, G, H, I, J, K, L, O, P, Q, R and S. The term -“Cucurbitacin” refers to group of Cucurbitacins along with their glyosidic forms mentioned above. Cucurbitacin G and H have same structures but differ from each other in the configuration of the hydroxyl group at position 24 which is not yet established. Cucurbitacin R was demonstrated to be 23, 24-dihydrocucurbitacin D (DHCD) hence, its description has been moved to the group of Cucurbitacin D. Similarly Cucurbitacin J and K differ from each other only in the configuration of hydroxyl group at position 24 which is yet to be determined. A special group of Cucurbitacins are called as momordicosides, named after their occurrence in Momordica charantia. Momordicosides have never been identified in any other plant species. The common feature of momordicosides is that C19 has been oxidized to an aldehyde group.
The saccharide linkage is generally present at C-2 (2-O-β-glycosides) in Cucurbitacin glycosides. Majority of Cucurbitacins are usually crystalline in nature or present in the form of needles at room temperature except Cucurbitacin H which is an amorphous solid. Most Cucurbitacins are soluble in petroleum ether, chloroform, benzene, ethyl acetate, methanol and ethanol, but are insoluble in ether. They are only slightly soluble in water. Cucurbitacins usually have absorption maxima for ultraviolet light wavelength (λ) between 228-234 nm (J. C. Chen, Chiu, Nie, Cordell, & Qiu, 2005). Nine cucumber genes are also responsible for biosynthesis of cucurbitacins and elucidated four catalytic steps. The transcription factors Bl (Bitter leaf) and Bt (Bitter fruit) that control this pathway in leaves and fruits, respectively. The Bi gene confers bitterness to the entire plant and is genetically related with an operon-like gene cluster, similar to the gene cluster involved in thalianol biosynthesis in Arabidopsis. Fruit bitterness requires both Bi and the dominant Bt (Bitter fruit) gene. Non bitterness of cultivated cucumber fruit is conferred by bt, an allele selected during domestication. Bi is a member of the oxidosqualene cyclase (OSC) gene family.
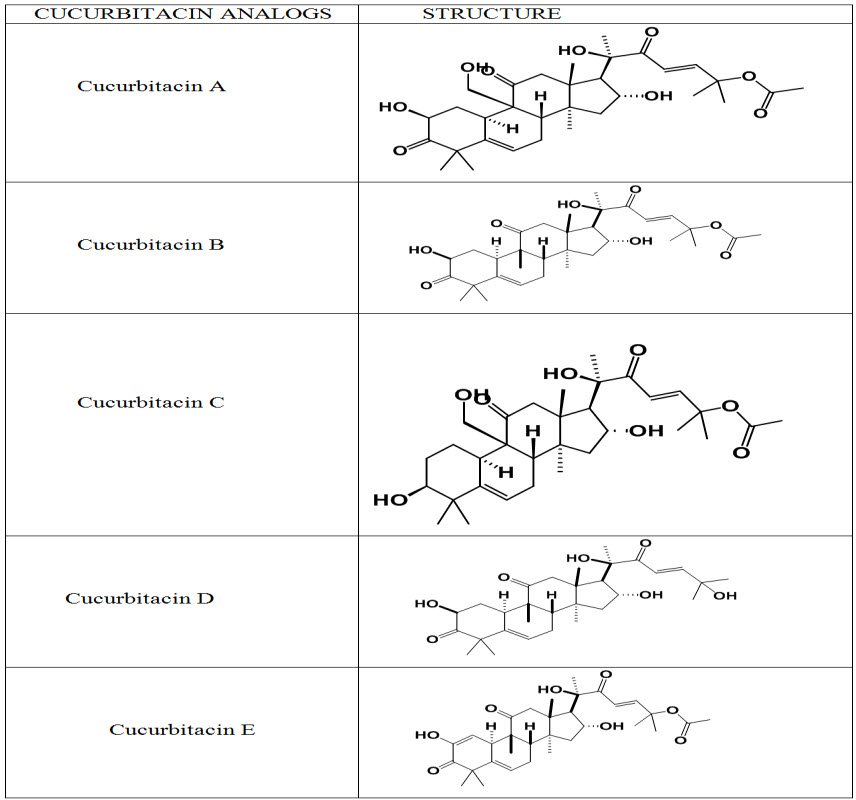
Table. 1: Structures of Cucurbitacins (Kaushik et al., 2015)
4. Anticancer activity of Cucurbitacin and its derivatives:
Cancer is a rapidly growing health threat. Cancer is a group of diseases in which the abnormal growth of cells with the potential spread to other parts of the body. Not all tumors are cancerous in the starting phase and do not spread to other parts of the body. The sign and symptoms include a lump, abnormal bleeding, prolonged cough, unexplained weight loss etc. these symptoms may indicate cancer (Kaushik et al., 2015). The cancer is a serious diseases cause 8.8 million people worldwide died from cancer in 2015. That is nearly 1 in 6 of all global deaths. Only 5-10% of all cancer cases can be attributed to genetic defects, whereas the remaining 90-95% has their roots in the environment and life style. The life style factors include cigarette smoking, diet, alcohol, sun exposure, environmental pollutants, infections, stress, obesity, and physical in activity (Teuscher, 1994). The evidence indicates that of all cancer related deaths, almost 25-30% are due to tobacco, as many as 30-35% are linked to diet, about 15-20% are due to infections, and the remaining percentage are due to other factors like radiation, stress, physical activity, environmental pollutants (Enslin, Joubert, & Rehm, 1956). It presents the biggest challenge to researchers and medical professionals and has been selected for various prevention and therapeutic strategies. The dietary intake of many vegetables and fruits has been found to reduce the risk of occurrence of cancer. Cancer is the deregulation of apoptosis that programmed cell death. Several promising targets for intervention is identified by studying the molecular abnormalities that tumor genesis, such as the signal transduction pathways that regulate apoptosis. In this perspective, cucurbitacins and its derivatives have become a new focus for cancer treatment because of their strong capability to inhibit several cancer. The target area of cucurbitacins and its derivatives through JAK/STAT pathway, Wnt signaling and MAPK pathway (Njoroge & Newton, 1994). Cucurbitacin and its derivatives are inhibited cancer growth via a wide range of mechanisms, including proapoptosis, induction of autophagy, cell cycle arrest, inhibition of cancer invasion, and migration. Cucurbitacins also modulate multiple intracellular signaling pathways.
It is interesting that each variant of cucurbitacins may trigger slightly different molecular signaling cascades in different cancer cell types to inhibit cancer growth and progress (Lavie, Willner, & Merenlender, 1964). Signal transducers and activators of transcription 3 (STAT3) and Janus kinase (JAK) signaling pathways are the main mechanisms for cucurbitacins to induce apoptosis to exert their potent anticancer effect. In addition, the ability of cucurbitacins to induce cell cycle arrest in G2/M phase mediated by various regulators is also an important way to fight against various cancers (Bartalis & Halaweish, 2005).
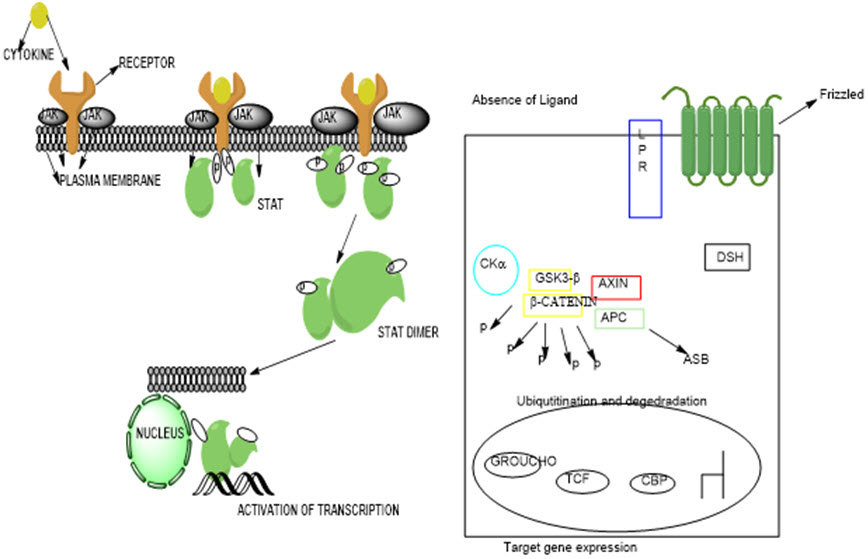
Fig. 3: (a) Mechanism of STAT signaling pathway leading to activation (Bowman, Yu, Sebti, Dalton, & Jove, 1999) (b) Canonical and noncanonical Wnt signaling pathway (Rao & Kühl, 2010)
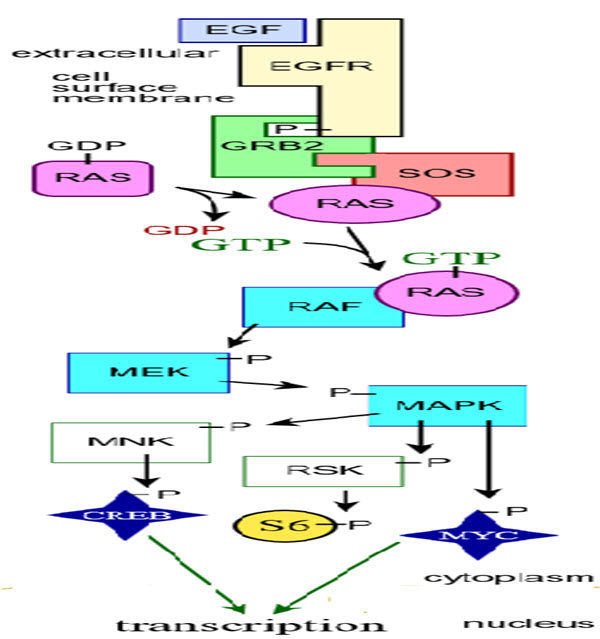
Fig. 4: Schematic diagram of MAP kinase cascades (Zhang & Liu, 2002)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Induction of Apoptosis:
Apoptosis is programed cell death that plays important role in the maintenance of body homeostasis. Abnormal apoptosis leads to the development and progression of many diseases, including neoplasms and autoimmune diseases. Cucurbitacins promote apoptosis through multiple molecular mechanisms. Cucurbitacin B, D, E, I, and IIa induce apoptosis in various types of cancer cells by inhibiting the STAT3 pathway. STAT3 is a transcription factor that controls gene expression through cross-talk with other transcription factors, such as β-catenin, hypoxia-inducible factor-1α (HIF-1α), nuclear factor-κB (NF-κB), c-myc, c-jun, etc and blocking STAT3 activation induces apoptosis (Gorski, Jaworski, Shannon, & Robinson, 1985). Cucurbitacin B have marked morphological changes as a marker for apoptosis, which include nuclear fragmentation, chromatin condensation, and formation of apoptotic bodies. However, it seems that the inhibition of STAT3 by cucurbitacin B is cell type-dependent and the antiproliferative effect of cucurbitacins is independent of the STAT3 pathway. Cucurbitacin B may be useful for both modulating the sensitivity of cancer cells to cytotoxic lymphocyte and promoting anticancer immunity through the pharmacological inhibition of the JAK2/STAT3 pathway (Oleszek, 2002). By suppressing the JAK/STAT pathway and decreasing Bcl-XL expression. Cucurbitacin B induces apoptosis in pancreatic cancer cells. These mechanisms indicate that inhibition of the JAK/STAT3 pathway by cucurbitacin B may be effective in cancer immunotherapy (Rice, Rymal, Chambliss, & Johnson, 1981). Moreover, in the cells of human colon adenocarcinoma, the cucurbitacin B-induced apoptosis is controlled by a reactive oxygen species (ROS)-dependent, but a STAT3-independent mechanism. Cucurbitacin B suppresses cancer progression by the inhibition of tumor necrosis factor (TNF)-induced expression of NF-κB-dependent anti-apoptotic proteins and transactivation activity of RelA/p65, which include TRAF1, TRAF2, c-IAP1, and c-IAP2. Furthermore, cucurbitacin B inhibits phosphorylation of ATP citrate lyase (ACLY), an important enzyme for cancer metabolism, leading to poly ADP ribose polymerase (PARP) cleavage, accumulation of sub-G0/G1 cell population and activation of caspase 3 and 7 (Gorski, Jaworski, Shannon, & Robinson, 1986). Cucurbitacin B also inhibits HER2 and integrin signaling in breast cancer. In addition, in breast cancer cells, cucurbitacin B promotes cell cycle arrest in G2/M phase and apoptosis by down-regulating Wnt-associated signaling molecules, including β-catenin, cyclin D1, galectin-3, c-Myc, and phosphorylated GSK-3β. Similarly, cucurbitacin E inhibits cell proliferation by suppressing Wnt/β-catenin signaling by up-regulating the cancer suppressor gene Menin. Cucurbitacin D activates the apoptotic pathway by suppressing STAT3 activity in breast cancer cells and cleaving fragments of procaspase-3 and -9 and PARP in human endometrial and ovarian cancer cells (Rubinfeld et al., 1993). Moreover, cucurbitacin D is considered as a potential anticancer drug treated on neurofibromatosis type 2 (Nf2)-deficient schwannoma as well as meningioma because of its proapoptotic effects through inhibiting expression of phospho-PRAS40 and phospho-Akt. Cucurbitacin IIa is a class of anticancer drug with decreasing survival in cancer cells by deranging actin cytoskeleton and mediating cells to go through PARP-mediated apoptosis via the suppression of survivin, which is a downstream protein in the JAK2/STAT3 pathway (Metcalf, Metcalf, & Rhodes, 1980). In pancreatic and ovarian cancer cells as well as squamous cell carcinoma, cucurbitacin E promotes the cleavage of caspase-3 and inhibits the phosphorylation of STAT3, but not extracellular signal-regulated kinase (ERK)-1/2. Apoptosis triggered by cucurbitacin E is accompanied by up regulating truncated BID and Fas/CD95 as well as down-regulation of Delta Psi (m), resulting in releases of apoptotic protease activating factor 1, cytochrome c and apoptosis inducing factor and serial activation of caspase-9, -8 as well as -3 (Lavie & Glotter, 1971). Cucurbitacin I, a JAK/STAT3 inhibitor, enhances apoptosis by inducing cleavages of PARP and caspase-3, -7, -8, and -9 in colon cancer. Cucurbitacin I also effectively suppresses the STAT3 pathway and expression of Bcl-2 and survivin in head and neck squamous cell carcinoma cells. With the role of a tyrosine kinase inhibitor, cucurbitacin I regulates proteasome degradation (Halaweish & Tallamy, 1993).
Induction of Autophagy:
Cucurbitacins, especially cucurbitacin B and I, trigger autophagosome formation as well as the accumulation and conversion from light chain 3-I to LC3II in many cell types mainly through increasing production of mitochondrial-derived ROS and subsequently activating ERK and JNK. Activation of the AMP-activated protein kinase/ mammalian target of the p70S6K pathway, but not the PI3K/Akt pathway, occurs in cucurbitacin I-induced autophagy in glioblastoma. Glioblastoma treated with cucurbitacins and also has a decreased level of HIF-1α (Bauer & Wagner, 1983)
Induction of Cell Cycle Arrest:
In human cells, cell cycle transition is controlled by holoenzymes consisting of both regulatory (cyclin) and catalytic (cyclin-dependent kinase (CDK)) subunits. CDK inhibitors CDK1, p21=Waf1 and p27KIP1 act as key regulators of the cell cycle by binding to CDK complexes and decreasing kinase activity. Cucurbitacins induce cell cycle arrest by modulating multiple signaling pathways (Bowman et al., 1999). Cucurbitacin B induces G2/M cell cycle arrest in various cancers, including osteosarcoma (OS) cell, non-small cell lung cancer, breast cancer, cutaneous squamous cell carcinoma, glioblastoma multiform, laryngeal squamous cell carcinoma and pancreatic cell (Turkson & Jove, 2000). In hepatocellular carcinoma cells, the Cucurbitacin B-induced cell cycle arrest in S phase is related to a significant decline in cyclin D1 and CDC2 levels, and no change in cyclin B1 level, which is different from the other cell lines, indicating that cucurbitacin B is probably cell specific in mediating inhibition of cell growth. In breast cancer cells, Cucurbitacin B arrests cell cycle by inhibiting telomerase via the down-regulation of human telomerase reverse transcriptase and c-Myc, as well as up-regulation of p21, Waf1 and p27Kip1 expression via the defective gene BRCA1. In addition, Cucurbitacin B down-regulates protein expression of cyclin B1 and CDC25C without reducing CDK1 expression in colon adenocarcinoma SW480 cells nor increasing p53 and p21 expression in neuroblastoma .In non-small lung cancer cells, Cucurbitacin B exerts its effect on cell cycle arrest through the down-regulation of CDK1, cyclin D1, and PCNA proteins. Cucurbitacin I decreases the expression of cell cycle proteins including cyclin B1, cyclin A, CDK1, and CDC25C in colon cancer cells (SW480). These results indicate that different cell types treated with cucurbitacins may not use the same mechanism to trigger cell cycle arrest (Bowman, Garcia, Turkson, & Jove, 2000). Furthermore, the microtubule cytoskeletons are relevant to cell motility and cytokinesis in various cancers.
The formation of cofilin-actin rods induced by cucurbitacin B is regulated by chronophin in dependent, but cofilin phosphatase Slingshot homolog 1-dependent hyper activation of cofilin in melanoma, leading to cancer cell growth arrest and inhibition of migration also, cucurbitacin B significantly changes cytoskeletal network in breast cancer and glioblastoma multiforme cells, which induces incorrect polymerization of microtubule network and fast morphologic changes (Dong et al., 2010). In the cells treated with cucurbitacin B, the G-actin pool is rapidly depleted and actin aggregates are quickly formed through the activation of a ROS dependent mechanism that regulates various cytoskeleton-regulatory proteins, ultimately leading to anticancer effects. Similar to cucurbitacin B, cucurbitacin IIa is a class of anticancer agent that inhibits cell survival in cancer by embroiling the actin cytoskeleton. Cell cycle arrest in G2/M phase was induced by cucurbitacin D and IIa in breast cancer. In Nf2-deficient meningioma and schwannoma cells, cucurbitacin D decreases cyclin A, B, and E and inhibits expression of phospho-PRAS40 and phosphoAkt (Duncan, Duncan, Alley, & Sausville, 1996). Furthermore, cucurbitacin D increases p21Waf1 as well as p27Kip1 proteins and decreases cyclin A and B in endometrial and ovarian cancer cells. In human bladder cancer T24, pancreatic, ovarian, and breast cancer cells, cell cycle arrest in G2/M phase induced by cucurbitacin E is related to a significant increase in the levels of p27, p21, and p53 along with inactivation of STAT3, CDK1, and cyclin B. Additionally, cucurbitacin E blocks cyclin B1/CDC2 complex formation and induces the expression of GADD45 in colorectal and human brain malignant gliomas cells, indicating that the delay of mitosis by cucurbitacin E is regulated by the overexpression of the GADD45 gene family (Duangmano et al., 2010).
Inhibition of Cancer Invasion and Migration:
Cancer metastasis is a complicated process including cell migration, adhesion, and proteolysis of the basement membrane (BM) and extracellular matrix (ECM). In particular, the matrix metalloproteinase (MMP) family accounts for degradation of the compounds in BM and ECM. Cucurbitacin B markedly suppresses cell migration and invasion induced by 12O-tetradecanoylphorbol 13-acetate by inhibiting the phosphorylation of Akt, p38, and ERK1/2, and the down-regulation of MMP-9. Cucurbitacin E suppresses breast cancer metastasis by disrupting Arp/23-dependent actin polymerization and blocking the Src/FAK/Rac/JNK/MMP signaling pathway. In nasopharyngeal carcinoma (NPC), Cucurbitacin I reduces the invasiveness of NPC cells and possesses potent anoikis-sensitization activity against NPC. Additionally, cucurbitacin I suppresses the activation of Rac1 in breast cancer cells through a Jak2independent and ROS-mediated mechanism that alters the balance between Rho and Rac, which are important factors for the metastatic dissemination and transformation of cancer cells (Jayaprakasam, Seeram, & Nair, 2003). Cucurbitacins have been reported mainly in the Cucurbitaceae, are a class of highly oxidized tetracyclic triterpenoids possess the biogenetically unusual 10α-cucurbit-5-ene [19(10 →19β) abeo-10α lanostane skeleton which are well known for their cytotoxic activity (Escandell et al., 2007). The ethanolic extract of air-dried fruits of C. pepo was subjected to chromatography to give cucurbita glycosides A and B. In vitro assay both compounds showed weak cytotoxic activity against HeLa cells with IC50 values of 17.2 and 28.5 µg/ml respectively (Park et al., 2004). Treatment with cucurbitacins B and E showed growth inhibition accompanied by apoptosis and cell cycle arrest in breast cancer cell lines (MDAMB-231 and MCF-7). They also modulated the expression of proteins involved in cell-cycle regulation in both of the estrogen-independent (MDA- MB-231) and estrogen-dependent (MCF-7) in human breast cancer cell lines (Yuan, Wahlqvist, He, Yang, & Li, 2006).
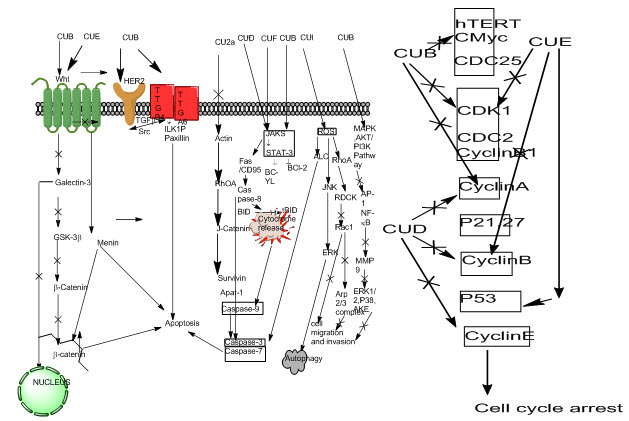
Fig. 5: Mechanisms of MAPK pathway for signaling network (Zhang & Liu, 2002)
Growth inhibition and cytotoxic effect of cucurbitacin B on breast cancer cell lines SKBR-3 and MCF-7 were attributed to G2/M phase arrest and apoptosis. Cucurbitacin B treatment inhibited Cyclin D1, c-Myc, and β-catenin expression levels, translocation to the nucleus of β-catenin and galectin-3. Western blot analysis showed increased PARP cleavage suggesting induced caspase activity and decreased mutagenic Wnt-associated signaling molecules galectin-3, β-catenin, c-Myc, and cyclin D1 with changes in phosphorylated GSK-3β levels (Esterbauer, 1993)[39]. (Cu B) and cucurbitacin E (Cu E) inhibit Wnt and STAT3 signaling pathways; Cu B inhibits HER2 and integrin signaling pathways and elevates intracellular level of ROS; cucurbitacin D (Cu D) inhibits STAT3 activation; and cucurbitacin IIa (Cu IIa) inhibits survivin. To trigger autophagy, cucurbitacin I (Cu I) increases intracellular level of ROS. To inhibit cell migration and invasion, Cu E and Cu I inhibit Rac1 activation, and Cu B inhibits phosphorylation of ERK1/2, p38, and Akt. To induce cell cycle arrest, Cu B, Cu D, and Cu E down-regulate protein expression of key regulators of cell cycle, including cyclin B1, etc.
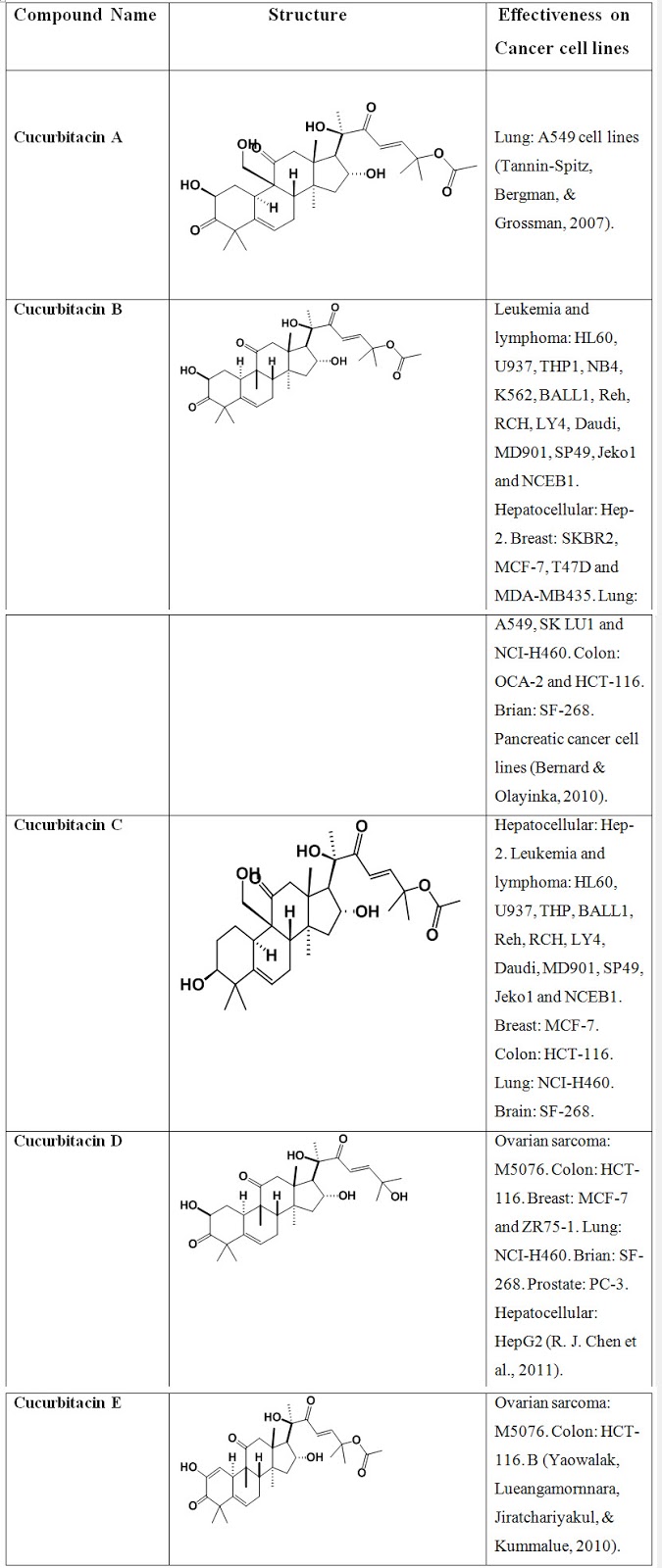
Table. 2: Compounds structure and their effectiveness on cancer cell lines
CONCLUSION:
In the past cucurbitacins have been viewed as toxic compounds with potential cytotoxic and anti-ecdysone effects. More recently, different researchers have established the selective role of some cucurbitacins as inhibitors of specific transcription factors. This new understanding of the activity and new data on the cytotoxicity of different cucurbitacins has led to the discovery of several interesting structural relationships which help avoid negative side effects, such as the presence of an acetoxyl group at C25, the double bond at C23, or the presence of a carbonyl or hydroxyl at C3, which can modify both the cytotoxicity and the pharmacological activity of cucurbitacins. The most interesting structures for obtaining new cytotoxic compounds are probably cucurbitacin B and E whereas the deacetyl-derivatives (e.g. cucurbitacin R) show the highest potential as the basis for a series of anti-inflammatory cucurbitacins. The different studies conducted on cucurbitacins and the similarity of the results shows that these compounds are a selective kind of JAK/STAT pathway inhibitor and that they may be highly interesting for treating cancers in which this pathway is implicated, such as human lung adenocarcinoma, glioblastoma multiform, nasopharyngeal carcinoma, or chronic lymphocytic leukemia Many studies have postulated that the concomitant use of different cucurbitacins, especially I and B, could improve the effects of gemcitabine, methotrexate, docetaxel, or cisplatin, which could allow for a possible reduction in dosage in order to avoid the high number of undesirable effects of these drugs. For these reasons, pumpkin seed show promise as anticancer agents, especially in combination with other therapeutic agents when chemo-resistance and radio-resistance constitute a serious problem for the patient. Cucurbitacins thus have a great potential for use as pharmacological agents in the prevention and treatment of cancer. In addition, they constitute a selective laboratory tool for studying compounds implicated in the JAK/STAT pathway and they will also be beneficial for database development and template design for future drug development. The purpose of this review is to gather the information about an anticancer potential related to these highly diverse group of Cucurbitacin which may be useful in future research.
REFERENCES:
1. Ali MS., Mukherjee S, Roy D, Pal, G, Makar S; (2018). Asparagus Racemosus, a Climbing Ayurvedic Medicinal Plant: Review on its Cultivation, Morphology and Medicinal Significance; PharmaTutor ; 6(12); 46-54.
2. Bartalis, J., & Halaweish, F. T. (2005). Relationship between cucurbitacins reversed-phase high-performance liquid chromatography hydrophobicity index and basal cytotoxicity on HepG2 cells. Journal of Chromatography B, 818(2); 159-166.
3. Bauer, R., & Wagner, H. (1983). Cucurbitacinhaltige Drogen. Dtsch Apoth Ztg, 123, 1313-1321.
4. Bernard, S. A., & Olayinka, O. A. (2010); Search for a novel antioxidant, anti-inflammatory/analgesic or anti-proliferative drug: Cucurbitacins hold the ace. Journal of Medicinal Plants Research, 4(25); 2821-2826.
5. Bowman, T., Garcia, R., Turkson, J., & Jove, R. (2000); STATs in oncogenesis. Oncogene, 19(21); 2474.
6. Bowman, T., Yu, H., Sebti, S., Dalton, W., & Jove, R. (1999); Signal transducers and activators of transcription: Novel targets for anticancer therapeutics. Cancer Control, 6(5); 1-7.
7. Chen, J. C., Chiu, M. H., Nie, R. L., Cordell, G. A., & Qiu, S. X. (2005); Cucurbitacins and cucurbitane glycosides: structures and biological activities. Natural product reports, 22(3); 386-399.
8. Chen, R. J., Jinn, T.-r., Chen, Y.-c., Chung, T.-y., Yang, W.-h., & Tzen, J. T. (2011); Active ingredients in Chinese medicines promoting blood circulation as Na+/K+-ATPase inhibitors. Acta Pharmacologica Sinica, 32(2); 141-51
9. Dinan, L., Harmatha, J., & Lafont, R. (2001); Chromatographic procedures for the isolation of plant steroids. Journal of chromatography A; 935(1-2); 105-123.
10. DINAN, L., WHITING, P., Girault, J.-P., LAFONT, R., DHADIALLA, S. T., CRESS, E. D., . . . LEPESANT, J.-A. (1997); Cucurbitacins are insect steroid hormone antagonists acting at the ecdysteroid receptor. Biochemical journal; 327(3); 643-650.
11. Dong, Y., Lu, B., Zhang, X., Zhang, J., Lai, L., Li, D., . . . Pang, X. (2010). Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2–STAT3 signaling pathway. Carcinogenesis, 31(12); 2097-2104.
12. Duangmano, S., Dakeng, S., Jiratchariyakul, W., Suksamrarn, A., Smith, D. R., & Patmasiriwat, P. (2010); Antiproliferative effects of cucurbitacin B in breast cancer cells: down-regulation of the c-Myc/hTERT/telomerase pathway and obstruction of the cell cycle. International journal of molecular sciences, 11(12); 5323-5338.
13. Duncan, K. L., Duncan, M. D., Alley, M. C., & Sausville, E. A. (1996); Cucurbitacin E-induced disruption of the actin and vimentin cytoskeleton in prostate carcinoma cells. Biochemical pharmacology, 52(10); 1553-1560.
14. Enslin, P., Joubert, F., & Rehm, S. (1956); Bitter principles of the Cucurbitaceae. III.—elaterase, an active enzyme for the hydrolysis of bitter principle glycosides. Journal of the Science of Food and Agriculture, 7(10); 646-655.
15. Escandell, J. M., Recio, M. C., Manez, S., Giner, R. M., Cerda-Nicolas, M., Gil-Benso, R., & Ríos, J.-L. (2007); Dihydrocucurbitacin B inhibits delayed type hypersensitivity reactions by suppressing lymphocyte proliferation. Journal of Pharmacology and Experimental Therapeutics, 322(3); 1261-1268.
16. Esterbauer, H. (1993); Cytotoxicity and genotoxicity of lipid-oxidation products. The American journal of clinical nutrition, 57(5); 779S-786S.
17. Gamlath, C. B., Gunatilaka, A. L., Alvi, K. A., & Balasubramaniam, S. (1988); Cucurbitacins of Colocynthis vulgaris. Phytochemistry, 27(10); 3225-3229.
18. Gorski, P., Jaworski, A., Shannon, S., & Robinson, R. (1985). Rapid TLC and HPLC test for cucurbitacins. Cucurbit Genetics Cooperative, 25.
19. Gorski, P., Jaworski, A., Shannon, S., & Robinson, R. (1986). Rapid TLC and HPLC quantification of cucurbitacin C in cucumber cotyledons. HortScience (USA).
20. Gry, J. (2006). Cucurbitacins in plant food: Nordic Council of Ministers.
21. Halaweish, F., & Tallamy, D. (1993); Quantitative determination of cucurbitacins by high performance liquid chromatography and high performance thin layer chromatography. Journal of Liquid Chromatography & Related Technologies; 16(2), 497-511.
22. Jayaprakasam, B., Seeram, N. P., & Nair, M. G. (2003); Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer letters, 189(1); 11-16.
23. Kaushik, U., Aeri, V., & Mir, S. R. (2015); Cucurbitacins–an insight into medicinal leads from nature. Pharmacognosy reviews; 9(17); 12-18
24. Kupchan, S. M., Meshulam, H., & Sneden, A. T. (1978); New cucurbitacins from Phormium tenax and Marah oreganus. Phytochemistry, 17(4); 767-769.
25. Lavie, D., & Glotter, E. (1971); The cucurbitanes, a group of tetracyclic triterpenes Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products 29 (pp. 307-362)
26. Lavie, D., Willner, D., & Merenlender, Z. (1964); Constituents of Citrullus colocynthis (L.) Schrad. Phytochemistry; 3(1); 51-56.
27. Makar, S., Saha, T., & Singh, S. K. (2018); Naphthalene, a versatile platform in medicinal chemistry: Sky-high perspective. European journal of medicinal chemistry; 161; 252-276
28. Metcalf, R. L., Metcalf, R. A., & Rhodes, A. (1980). Cucurbitacins as kairomones for diabroticite beetles. Proceedings of the national academy of sciences, 77(7); 3769-3772.
29. Nag M, Mukherjee S, Roy D, Paul RK, Ali MS; (2018); Extraction and Chemical tests on Cicer Arietinum seed collected from North Bengal Region of West Bengal, India; PharmaTutor; ; 6(10); 1-4
30. Njoroge, G. N., & Newton, L. E. (1994); Edible and poisonous species of Cucurbitaceae in the Central Highlands of Kenya. Journal of East African Natural History, 83(2); 101-115.
31. Oleszek, W. (2002); Chromatographic determination of plant saponins. Journal of chromatography A; 967(1); 147-162.
32. Park, C. S., Lim, H., Han, K. J., Baek, S. H., Sohn, H. O., Lee, D. W., . . . Kwon, N. S. (2004); Inhibition of nitric oxide generation by 23, 24-dihydrocucurbitacin D in mouse peritoneal macrophages. Journal of Pharmacology and Experimental Therapeutics; 309(2); 705-710.
33. Rao, T. P., & Kühl, M. (2010); An updated overview on Wnt signaling pathways: a prelude for more. Circulation research, 106(12); 1798-1806.
34. Rice, C., Rymal, K., Chambliss, O., & Johnson, F. (1981); Chromatographic and mass spectral analysis of cucurbitacins of three Cucumis sativus cultivars. Journal of Agricultural and Food Chemistry, 29(1); 194-196.
35. Rubinfeld, B., Souza, B., Albert, I., Muller, O., Chamberlain, S. H., Masiarz, F. R., . . . Polakis, P. (1993); Association of the APC gene product with beta-catenin. Science, 262(5140); 1731-1735.
36. Stuppner, H., & Moller, E. (1993); Cucurbitacins with unusual side chains from Picrorhiza kurroa. Phytochemistry, 33(5); 1139-1145.
37. Stuppner, H., Müller, E. P., & Wagner, H. (1991); Cucurbitacins from Picrorhiza kurrooa. Phytochemistry; 30(1); 305-310.
38. Tannin-Spitz, T., Bergman, M., & Grossman, S. (2007); Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochemical and Biophysical Research Communications; 364(1); 181-186.
39. Teuscher, E. (1994); und Lindequist, U.(eds): Biogene Gifte-Biologie, Chemie, Pharmakologie: Gustav Fischer Verlag.
40. Turkson, J., & Jove, R. (2000); STAT proteins: novel molecular targets for cancer drug discovery. Oncogene; 19(56); 6613-26
41. Yaowalak, U.-p., Lueangamornnara, U., Jiratchariyakul, W., & Kummalue, T. (2010). Immunosuppressive effects of Cucurbitacin B on human peripheral blood lymphocytes. Journal of Medicinal Plants Research, 4(22); 2340-2347.
42. Yuan, G., Wahlqvist, M. L., He, G., Yang, M., & Li, D. (2006); Natural products and anti-inflammatory activity. Asia Pacific journal of clinical nutrition; 15(2); 143-52
43. Zhang, W., & Liu, H. T. (2002). MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell research, 12(1), 9.
44. Zheng, C.-H., Fu, H.-W., & Pei, Y.-H. (2007); A new cucurbitacin from Bolbostemma paniculatum Franguent: Note. Journal of Asian natural products research; 9(2); 187-190.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









