{ DOWNLOAD AS PDF }
ABOUT AUTHOR:
Jay Shah1, Nazima Mirza2, Vivek Patel3
1Department of Clinical Pharmacy, A.R.College of Pharmacy and G.H.Patel Institute of Pharmacy,
Vallabh Vidyanagar, Gujarat, India.
2Department of Pharmacology, Pramukh Swami Medical College, Karamsad, Gujarat
3Department of Orthopedic, Shree Krishna Hospital, Karamsad, Gujarat
shahjayg@yahoo.com
ABSTRACT
Objectives: Osteoarthritis (OA) is the most common form of arthritis which has a growing number of patients and having major impact on the quality of life. According to modes of action the drugs for OA are divided into two groups. First group, NSAIDs which provide symptomatic relief but have no influence on progress of the disease and shows higher adverse events. The second group of drugs acts as disease modifying agents. Recent data from clinical trials have demonstrated that agents like Diacerein & Oxaceprol specifically block key disease mechanisms & effectively retard the progression of structural changes in knee OA patients. The study was undertaken to compare the efficacy & safety of Oxaceprol and Diacerein in the patients of Osteoarthritis of Knee joints.
Experimental/ Computational work done: Total 60 patients(4 withdrawn) suffering from mild to moderate OA of knee joint were included from outpatient orthopedic department of Shree Krishna hospital, Karamsad. Twenty eight patients received diecerein(50 mg b.i.d.) and 28 patients were given Oxaceprol(200 mg t.i.d.) for 10 days and patient responses were measured using VAS and Lequesne scale, before and after drug treatment. Suspected adverse event was evaluated by filling ADR form. Ethical committee approval and patient consents were obtained prior to the beginning of the study.
Results and discussion: The VAS Scale was reduced from 7.321 ± 0.9449 to 4.75 ± 1.146 (Mean±SD) for Diacerein and 6.821 ±1.1564 to 4.321±1.1564 for Oxaceprol. The Lequesne scale was reduced from 6.0±1.054 to 4.21±1.0923 for Diacereine and 6.0±0.8819 to 3.839±1.0475 for Oxaceprol. Both the results were found to be statistically significant. Difference in VAS and Lequesne scale between the Diacerein and Oxaceprol were 0.757 and 0.099 respectively (p >0.05). There was not any Adverse Event reported.
Conclusions: Both the drugs, Diacerein and Oxaceprol, individually and equally effective in Osteoarthritis condition. Both Diacerein and Oxaceprol are found to be safer.
INTRODUCTION:
The most common form of joint disease, osteoarthritis (OA) represents a major cause of morbidity and disability, particularly in the second half of life. The burden of this disease has been steadily gaining importance in the last few decades with the aging of the world's population.1
Osteoarthritis (OA) is a slowly progressive disease of unknown cause and obscure pathogenesis. The disease appears to originate in the cartilage and changes in this tissue are progressively severe with advancing age. In OA, both mechanical and enzymatic factors are involved in cartilage matrix degradation. In addition to cartilage, IL-1 affects the function of other articular tissues. IL-1 is considered a major factor of chondrocyte and synoviocyte activation since it induces the synthesis of neutral metalloproteases and promotes the destruction of macromolecules in the matrix and is amply involved in cartilage degradation and in synovial inflammation.2
Osteoarthritis (OA) is the most common joint disorder. Symptomatic knee OA occurs in 10% men and 13% in women aged 60 years or older. The number of people affected with symptomatic OA is likely to increase due to the aging of the population and the obesity.3
Across the globe more than 50% of people over 65 years of age have radiological evidence of osteoarthritis. In the treatment for osteoarthritis, the major goals of treatment are pain control with minimal adverse effects, maintenance or improvement of joint mobility and function, and improved health related quality of life.4
The current treatment of osteoarthritis is primarily focused on symptomatic relief by use of rapid action drugs (analgesics and NSAIDs) and newer cycloxygenase (COX-2) specific inhibitors.
Osteoarthritis was classified as a mechanical wear and tear disorder of articular cartilage for which only pain modifying therapies such as analgesics were prescribed with little scientific attention focused on modification of course of disease leading to musculoskeletal disability and affecting quality of life.
Analgesics/NSAIDs use increases the risk of upper gastrointestinal adverse effects and does not effect the underlying pathogenesis of articular diseases , thus have minimal role in modifying disease course and improving quality of life. Although COX -2 inhibitors have reduced incidence of gastrointestinal adverse events but may have significant renal and cardiovascular toxicities. Hence, there is continuous search of new and better drug for OA.5
Based on the fact, that a central hallmark in the development of OA is the progressive destruction of the joint tissue, a reasonable approach to the development of an effective medical treatment seems to be the search for drugs with the ability to slow down this process, or may be even arrest it completely. These drugs, termed disease modifying osteoarthritis drugs (DMOADs).
There are various studies available which shows these group of drugs gives better result in Osteoarthritis condition. Among these Diacerein and Oxaceprol found to be better as compared to classical NSAIDs in terms of efficacy and safety.
One study by Dilip kumar et al.(2010) shows Diacerein is very useful, disease modifying drug for Osteoarthritic patients bringing about structural and functional changes in the joints with less adverse effects.6
Diacerein or diacetylrhien (4, 5- diacetoxy-9, 10-dihydro- 9, 10 di-oxo-2 anthracene carboxylic acid) is a new anti-inflammatory, analgesic and antipyretic drug. It is an oral agent that has been developed specifically for the treatment of osteoarthritis. It has a novel mode of action that differentiates it from NSAIDs and other conventional form of drug therapy. Clinical trials have shown that diacerein is highly effective in relieving the symptoms of osteoarthritis. Findings from in vitro study have demonstrated that in contrast to NSAIDs, diacerein does not inhibit the synthesis of prostaglandins. As a result of this characteristic, diacerein shows no gastrointestinal toxicity.
Other study by Ashish prashad et al.(2012) shows, Oxaceprol is well-established drug for symptomatic treatment of joint disease. Recent preclinical and clinical publications demonstrate that it is a novel class of anti-inflammatory agent with better safety profile than classical NSAIDs. In comparison to diclofenac, oxaceprol was shown to be equally efficacious, yet, it exhibited better safety profile. Oxaceprol, should provide a viable therapeutic alternative to the patients by virtue of its cartilage protective property and better safety profile in the management of osteoarthritis.4
Oxaceprol (N-acetyl-L-hydroxyproline)is an amino acid derivative. Instead of inhibiting the synthesis of prostaglandins oxaceprol prevents leukocyte infiltration into the joints, thus inhibiting an early step of inflammatory cascade and presenting a novel class of anti-inflammatory agents.
Oxaceprol affects leucocyte rolling and adherence. The reduction in leucocyte adherence helps to protect the integrity of the endothelium to the extent that the macromolecular leakage is reduced. Oxaceprol is shown to be effective in inhibiting leucocyte emigration. In vivo studies have exhibited that treatment with oxaceprol causes a significant reduction in the arthritic swelling of the knee joints. Oxaceprol is devoid of COX inhibitory activity.
Most of the study found comparision with classical NSAIDs. Till now, no comparative study held among this type of disease modifying group of drug, So the present study entitled, “A Comparative study for Safety and Efficacy of Oxaceprol and Diacerein in Osteoarthritis of Knee joints” was undertaken with the aim and objective to check which drug shows better relief in mild to moderate osteoarthritis condition as compared to other and to evaluate which drug is more safer on basis of adverse drug reaction reported.
Materials and Methods
A prospective study was taken up for a period of 4 months, during December 2013 to March 2014 with 60 Patients, diagnosed as suffering from Osteoarthritis of knee was included in the study in accordance of inclusion and exclusion criteria from Orthopedic Department of Shree Krishna Hospital, Karamsad.
HREC Approval
Submission of protocolwith ICF and CRF to Human Research Ethics Committee (HREC) at H.M.Patel Centre for Medical Care & Education, Karamsad on 15th Sept. 2013, and Approval of protocol was given on 11th Jan. 2014
Inclusion and Exclusion Criteria
· Inclusion criteria:
- Patients, Newly diagnosed as suffering from Osteoarthritis of knee.
- Under Mild to Moderate Osteoarthritis condition (by Lequesne scale).
- Sex: Male and Female.
- Age: Above 30 years.
· Exclusion criteria:
- Patients allergic to Oxaceprol and/or Diacerein.
- Patients with history of or presence of Peptic Ulcer or Gastric Bleeding.
- Pregnant and Lactating women.
Methodology of the study
· Study Design: Prospective, Comparative, Observational study.
· Sample Size: 60 (30 patients treated with Oxaceprol, 30 patients treated with Diacerein.)
· Study Duration: Maximum 4 months.
· Treatment Duration: 10 days.
· Study Drug:
- Oxaceprol (Adult – 200 mg tid.)
* Diacerein (Adult – 50 mg bid.)
· Treatment was allocated by simple randomization method.
· Before enrolling patient into study, Informed Consent was taken from patients in prescribed from in the language they understand.
· After obtaining approval, 60 patients of any sex diagnosed as suffering from Osteoarthritis and who satisfy the Inclusion/Exclusion criteria were enrolled in the study after obtaining their Consent on Informed Consent Form (ICF).
· Demographic data (including Age, Sex, Weight, Personal history like Smoking and Alcohol etc), with VAS and LEQUESNE Scale were obtained from the patients and were documented on the structured Case Record Forms(CRF) prepared for the study.
· Both the Drug prescribed for 10 days with paracetamol (as an analgesic) 500mg(wt less than 50) or 650mg(wt greater than 50) bid.
· Data Collection: Patients datawere documented on the structured Case Record Forms.
· Patients responses were recorded before and after drug treatment (on 1st and 10th day) with study proforma.
· Patients responses were measured using VAS(Visual Analogue Scale) and Lequesne Scale.
* Visual Analogue Scale7
The visual analogue scale (VAS) is a horizontal line, typically 10 cm in length, anchored by textual descriptors and/or pictures at each end. An endpoint descriptor such as ‘no pain’ (a score of 0) is marked at the left end, and ‘worst pain imaginable’ or ‘worst possible pain’ (a score of 10) is marked at the right end. The patient is asked to indicate a point along the line that represents their perception of their pain. The VAS score out of 10 is determined by measuring the distance in cm from the left end of the line to the point that the patient.
* Lequesne Scale8
Lequesne scale used to assess the effectiveness of therapeutic interventions. It is a measure of 3 different parameters (I)Pain or Discomfort, (II)Maximum Distance Walked and (III)Activities of Daily Living. As shown in Annexure II each parameter has different points with minimum 0 to maximum of 8, then total points for all parameter were taken as index if severity score.
Plan of statistical analysis
In the present study the data have subjected to Paired and Independent t-test as applicable. P values less than 0.05, 0.01 and 0.001 were considered as significant, very significant and highly significant respectively.
RESULTS AND DISCUSSION
A total of sixty patients were enrolled in the study. Of these, four patient were withdrawn from the study, two from Diacerein group (one patient consulted other doctor and another patient had not taken the drug) and two from Oxaceprol group (one patent was unable to contact and another patient had not taken drug), So here data of fifty six patient were included for result.
Analysis of VAS and Lequesne Scale for Diacereinand Oxaceproldrug using Paired T-Test
Table-1: Paired T-Test table
|
|
|
|
Mean |
Std. Deviation |
Std. Error Mean |
P value |
Inference |
|
Diacerein |
VAS scale |
Before |
7.321 |
0.9449 |
0.1786 |
2.48 e-14 |
Extremely Significance |
|
After |
4.75 |
1.1746 |
0.2220 |
||||
|
Lequesne scale |
Before |
6.00 |
1.0540 |
0.1992 |
5.9 e-12 |
Extremely Significance |
|
|
After |
4.214 |
1.0923 |
0.2064 |
||||
|
Oxaceprol |
VAS scale |
Before |
6.821 |
1.1564 |
0.2185 |
1.03 e-16 |
Extremely Significance |
|
After |
4.321 |
1.1564 |
0.2185 |
||||
|
Lequesne scale |
Before |
6.00 |
0.8819 |
0.1667 |
4.74 e-14 |
Extremely Significance |
|
|
After |
3.839 |
1.0475 |
0.1976 |
Figure- 1: Mean score for both drug
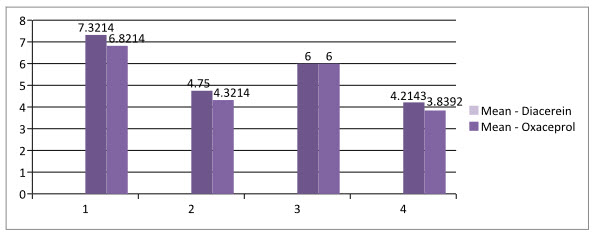
The VAS Scale was reduced from 7.321±0.9449 to 4.75±1.146 (Mean±SD) for Diacerein and 6.821±1.1564 to 4.321±1.1564 for Oxaceprol. The Lequesne scale was reduced from 6.0±1.054 to 4.21±1.0923 for Diacerein and 6.0±0.8819 to 3.839±1.0475 for Oxaceprol. Both the results were found to be statistically significant.
Above Table 1 and Figure 1 shows analysis of individual scale for both the drug by paired t-test. Diacerein drug shows reduction in VAS scale by 2.57 which is higher than the observation made by Dr B Medhi (2007)wherein he reported that VAS scale reduces by 1.5 level.9 In case of Oxaceprol drug, it reduces VAS scale 2.50 which is quite higher than the observation made by Kruger (2007)wherein he reported10 that VAS scale reduces by 1, Where as Ashish Prasad (2012) reported that Lequesne scale reduces4 to 2.50 which is slightly higher than that of our study which is 2.17.
The higher reduction found in our study may be due to concurrent administration of Paracetamol with study drugs. HREC suggested that we cannot deprive the patient with available therapy so paracetamol was given to every patients in the study to remove the bias, Other reason could be due to inclusion of Patients suffering from mild to moderate OA of knee. We could have got the different result if we would have included all patients having even severe form of OA and other comorbid condition.
In all cases Null hypothesis is rejected. i.e. both the drug Diacerein and Oxaceprol, individually shows effective reduction in VAS and Lequesne both scale after treatment so that, Statistically we can say that both the drug Diacerein and Oxaceprol are effective in OA Knee condition.
Analysis of Mean reduction in score of VAS and Lequesne Scale between Diacereinand Oxaceproldrug using Independent T-Test
Table-2: Independent T-Test table
|
Scale |
Drug |
Mean reduction in score |
Std. Deviation |
Std. Error Mean |
P value |
Inference |
|
Diff. in VAS scale |
Diacerein |
2.57142 |
0.95949 |
0.18132 |
0.75700 |
No significant difference |
|
Oxaceprol |
2.5 |
0.7453 |
0.1408 |
|||
|
Diff. in Lequesne scale |
Diacerein |
1.7857 |
0.8435 |
0.1594 |
0.09905 |
No significant difference |
|
Oxaceprol |
2.1607 |
0.8283 |
0.1565 |
Figure- 2: Difference in scale
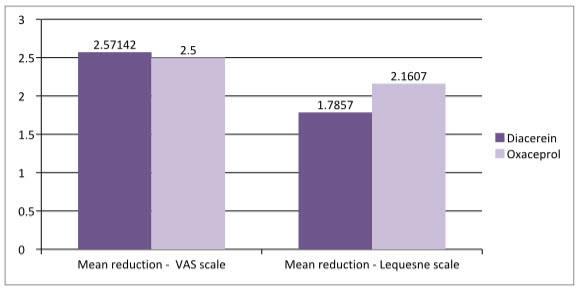
As shown in figure 2, the mean reduction in Lequesne score of Oxaceprol is 2.1607 which is higher than that of Diacerein which is1.7857, that shows clinically Oxaceprol found to be better than Diacerein but further studies may required.
Table 2 shows, statistically both the drug are equally effective in Osteoarthritis and both the drug Diacerein and Oxaceprol significantly reduces pain score, so stastistically we cann’t conclude that one drug is superior over other, so that both are equally effective in Knee Osteoarthritis condition.
Safety Analysis:
Including all 56 patients, we hadn’t received any adverse event complain from any of the patient for both the drug so that, no any Adverse Drug Reaction form is noted and concluded that both the drug are safe for patient.
LIMITATIONS:
The study is constrained by some limitations. (a) The sample size is small (n=56). (b) Treatment duration(10 days) is less to conclude result. (c) Due to Presence of available therapy for pain control Paracetamol was given along with both the drug.
However, these limitations of the study do not underscore the importance of the findings. The observations made from the study, at least, provide the links for future similar studies while providing the base line information for future comparison.
CONCLUSION:
Both the drug Diacerein and Oxaceprol, statistically found to reduce VAS and Lequsne scale individually, so both the drug individually effective in Osteoarthritis of knee joint. The mean reduction in score was found to statistically insignificant between the drug so, both the drug are equally effective in Osteoarthritis of knee joint.No adverse event reported for both the drugs so, on the basis of adverse event both the drug found to be safer in Osteoarthritis of knee joint.
REFERENCE ID: PHARMATUTOR-ART-2191
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 6 Received On: 01/03/2014; Accepted On: 25/04/2014; Published On: 01/06/2014 How to cite this article: J Shah, N Mirza, V Patel; A Comparative study for Safety and Efficacy of Oxaceprol and Diacerein in Osteoarthritis of Knee Joints; PharmaTutor; 2014; 2(6); 108-114 |
REFERENCES
1. Johanne Martel, Lukas Wildi, Jean Pelletier, “Future therapeutics for osteoarthritis” Bone. 2012, 51, 297–311.
2. Michael Yaron, Idit ShirazI, “Anti-interleukin-1 effects of diacerein and rhein in humanosteoarthritic synovial tissue and cartilage cultures” Osteoarthritis and Cartilage. 1999, 7, 272–280.
3. Yuqing Zhang, Joanne Jordan, “Epidemiology of Osteoarthritis” Clin Geriatr Med. 2010, 26(3), 355–369.
4. Ashish Prasad, Manish Mahajan and Alok Chaturvedi, “ Oxaceprol in the Management of Osteoarthritis”, The Indian Practitioner. 2012, 65(10), 631-634.
5. Annil Mahajan, Kulbir Singh, Vishal Tandon, Sudesh Kumar and Hardeep Kumar, “Diacerein : A New Symptomatic Slow Acting Drug for Osteoarthritis”, JK Science. 2006, 8(3), 173-175.
6. Dilip Kumar Renapurkar and Shobana Mathur, “Evaluation of Efficacy and Safety of Diacerein in Osteoarthritis of Knee Joint”, International Journal of Pharma and Bio Sciences. 2010, 1(3), 1-12.
7. Wewers ME & Lowe NK, “A critical review of visual analogue scales in the measurement of clinical phenomena”, Research in Nursing and Health. 1990, 13, 227-236.
8. Lequesne MG, “The algofunctional indices for hip and knee osteoarthritis”, J Rheumatol. 1997, 24, 779-781.
9. Dr B Medhi, Dr PK Singh, Dr A Prakash, “Diacerein: A New Disease Modulating Agent in Osteoarthritis”, IJPMR. 2007, 18, 48-52.
10. K. Kruger, M. Klasser, J. Mossinger, U. Becker, “Oxaceprol – a randomised, placebo-controlled clinical study in osteoarthritis with a non-conventional non-steroidal anti-inflammatory drug”, Clinical and Experimental Rheumatology. 2007, 25, 29-34.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









