 ABOUT AUTHORS:
ABOUT AUTHORS:
RANI SHALU1*, SAROHA KAMAL1, NANDA SANJU2
1Institute of Pharmaceutical Sciences,
Kurukshetra University, Kurukshetra 136119, Haryana, India.
2Department of Pharmaceutical Sciences,
MDU, Rohtak 124001,
Haryana, India.
ABSTRACT
The aim of the present study was to investigate the in-vitro release properties of Ziprasidone hydrochloride monohydrate from different topical vehicles. By the unique advantages over the traditional drug delivery, transdermal drug delivery is becoming increasingly important and has got a vital interest in pharmaceutical industries. An in vitro release experiment was designed to reveal the rate of release of ziprasidone hydrochloride monohydrate from four different topical vehicles: (i) cream; (ii) a gel; (iii) an ointment (iv) pronoisomal gel. In vitro release of ziprasidone hydrochloride monohydrate from the four bases was monitored spectrophotometrically at a wavelength of 318 nm. In vitro release study results showed that the release of drug from vehicles ranks according to the following order: gel> proniosomal gel> cream> ointment. Gel base showed considerably higher drug release than other vehicles. Five types of chemical enhancers was used in the study and among them tulsi oil was the best enhancer. As we increase the concentration of chemical enhancer the release of drug also increases. By monitoring and attempting to explain the many possible reasons for the different rates of drug release from the vehicles, it was hope that the experiment would confer essential information concerning factors affecting the release of drugs from topical formulations.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1253
INTRODUCTION
Atypical antipsychotics are widely used in the treatment of schizopherenia and schizoaffective disorder [1]. On oral administration of ziprasidone hydrochloride monohydrate it showed 50% bioavilability, 99.9% plasma protein binding, hepatic first pass metabolism etc these potential parameters are overcome by transdermal drug delivery system or topical administration of drug [2,3]. Skin is one of the most readily accessible organs on human body for topical administration and is main route for topical drug delievery system. For topical formulation to be effective, it must penetrate the skin, only when the drug has entered the lower layers of skin it can be absorbed by blood and transported to the site of action. The stratum corneum provides the greatest resistance to penetration and it is the rate limiting step in percutaneous absorption. The permeation of drugs through skin can be enhanced by physical methods such as mechanical disruption, electrical disruption, and chemical modification or by the use of chemical penetration enhancers. Chemical penetration enhancers modify barrier properties of stratum corneum and hence increase drug permeability across skin [4, 5]
Tulsi is a widely grown, sacred plant of India and it belongs to the labiateae family. Leaf contains eugenol (volatile oil), ursolic acid (triterpenoid) and rosmarinic acid (phenylpropanoid). Other active compounds include caryophyllene and oleanolic acid. Seeds contain fixed oils having linoleic acid and linolenic acid. It has long history of use in ayurvedic system of medicine to treat various ailments without any noticeable toxicity. In the present study, attempts have been made to explore the penetration enhancing activity of tulsi oil [6].
MATERIALS AND METHODS
Ziprasidone hydrochloride monohydrate was received as a gift sample from New Delhi, India. Carbopol, liquid paraffin, triethanolamine, cholesterol and other chemicals were of analytical grade and used without further purification.
Method of preparation of bases
1 GEL (G): Carbopol gel was prepared by mixing carbopol 940 with distilled water, ethanol and drug (mixture I). Mixture II was prepared by mixing triethanolamine, ethanol distilled water and enhancer. Then add mixture II drop by drop to mixture I and the gel was prepared7.
2 CREAM (C):Grate white beeswax. Melt it with liquid paraffin and raise temperature to 700C. Dissolve borax in water and heat to 700C. Gradually add the solution to the melted mixture and stir until cold. Transfer to a well closed container7.
3 OINTMENT (O):Vaseline was melted on a water bath maintained at 600C and other component was warmed to the same temperature. The mixture was then removed from the water bath and stirred together until it congealed8.
4 PRONIOSOMAL GEL (PG):Proniosomal gel was prepared by a coacervation separation method. Precisely weighed amount of surfactant, lecithin, cholesterol and drug were taken in a clean and dry wide mouthed glass vial of 5 ml capacity was added to it after warming, all the ingredients were mixed well with glass rod; the open end of the glass bottle was covered with a lid to prevent the loss of solvent from it and warmed over water bath at 60-700C for about 5 min until the surfactant mixture was dissolved completely. Then the aqueous phase (0.1% glycerol solution) was added and warmed on a water bath till a clear solution was formed which was converted in to proniosomal on cooling. The gel so obtained was preserved in the same glass bottle in dark conditions for characterization9.
Permeation enhancers:Different permeation enahncers was used in this study such as tulsi oil, oleic acid, PEG 6000, span 40, DMSO.
Table 1: Composition of carbopol gel with ziprasidone by using different concentrations of different enhancers
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
G1 |
G2 |
G3 |
G4 |
G5 |
G6 |
G7 |
G8 |
G9 |
G10 |
G11 |
G12 |
G13 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
Carbapol 940 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|
Distilled water |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
Ethanol |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
|
Triethanolamine |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
|
Ethanol |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
|
Distilled water |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
|
DMSO |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
|
PEG 6000 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
|
Oleic acid |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
G14 |
G15 |
G16 |
G17 |
G18 |
G19 |
G20 |
G21 |
G22 |
G23 |
G24 |
G25 |
G26 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
Carbapol 940 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|
Distilled water |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
Ethanol |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
18.75 |
|
Triethanolamine |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
|
Ethanol |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
6.25 |
|
Distilled water |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
49.3 |
|
DMSO |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
PEG 6000 |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Oleic acid |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
Table 2: Composition of cream with ziprasidone by using different concentrations of different enhancers
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
C7 |
C8 |
C9 |
C10 |
C11 |
C12 |
C13 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
White bees wax |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Liquid paraffin |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
|
Borax |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Water |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
|
DMSO |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
|
PEG 6000 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
|
Oleic acid |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
C14 |
C15 |
C16 |
C17 |
C18 |
C19 |
C20 |
C21 |
C22 |
C23 |
C24 |
C25 |
C26 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
White bees wax |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
Liquid paraffin |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
60 |
|
Borax |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Water |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
|
DMSO |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
PEG 6000 |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
-
|
- |
- |
|
Oleic acid |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
Table 3: composition of ointment with ziprasidone by using different concentration of different enhancers
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
O1 |
O2 |
O3 |
O4 |
O5 |
O6 |
O7 |
O8 |
O9 |
O10 |
O11 |
O12 |
O13 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
Vaseline |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
|
Liquid paraffin |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
|
DMSO |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
|
PEG 6000 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
|
Oleic acid |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
O14 |
O15 |
O16 |
O17 |
O18 |
O19 |
O20 |
O21 |
O22 |
O23 |
O24 |
O25 |
O26 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
Vaseline |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
|
Liquid paraffin |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
|
DMSO |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
PEG 6000 |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
-
|
- |
- |
|
Oleic acid |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
Table 4: composition of proniosomal gel with ziprasidone by using different concentration of different enhancers
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
PG1 |
PG2 |
PG3 |
PG4 |
PG5 |
PG6 |
PG7 |
PG8 |
PG9 |
PG10 |
PG11 |
PG12 |
PG13 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
Span 60 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
|
Span 80 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
|
Lecithin |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
|
Cholesterol |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
|
Alcohol |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
|
Water |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
|
DMSO |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
|
PEG 6000 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
|
Oleic acid |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Ingredients (gm) |
Formulation Code |
||||||||||||
|
|
PG14 |
PG15 |
PG16 |
PG17 |
PG18 |
PG19 |
PG20 |
PG21 |
PG22 |
PG23 |
PG24 |
PG25 |
PG26 |
|
Ziprasidone |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
Span 20 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
|
Span 40 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
29 |
|
Lecithin |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
|
Cholesterol |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
5.9 |
|
Alcohol |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
17.7 |
|
Water |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
11.8 |
|
DMSO |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Tulsi oil |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
PEG 6000 |
15% |
20% |
25% |
- |
- |
- |
- |
- |
- |
- |
-
|
- |
- |
|
Oleic acid |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
- |
- |
- |
- |
- |
|
Span 40 |
- |
- |
- |
- |
- |
- |
- |
- |
5% |
10% |
15% |
20% |
25% |
Procedure of standard curve
Dissolve 2.38g of disodium hydrogen phosphate, 0.19g of potassium dihydrogen phosphate, and 8g of sodium chloride to produce 1000 ml.
A 100µg/ml stock solution of ziprasidone HCl was prepared in saline buffer pH 7.4 by first dissolving 10 mg of the drug in 10 ml of methanol and then, making up the final volume with saline buffer pH 7.4.
The λmax of ziprasidone hydrochloride monohydrate was determined by scanning suitable dilutions with high correlation coefficient. From the stock solution, various standard dilutions were made to obtain 10, 20, 30 and 100µg/ml and their respective absorbance values were measured at fixed λmax 318 nm.
[adsense:468x15:2204050025]
Procedure of in vitro release studies
The dissolution test was performed using standard USP apparatus II with some medications by using modified paddle using phosphate buffer of pH 7.4. The dissolution medium was 900 ml phosphate buffer pH 7.4. The temperature was maintained at 37±0.50C. The rotation speed was 50 rpm. Samples of 10 ml was withdrawn at predetermined time intervals 0.5h, 1h, 2h, and up to 12hrs and replaced with fresh and preheated 37±0.50C buffer solution each time. Samples were measured spectrophotometrically at 318nm. The amount released was calculated from regression line of the standard curve developed in the same medium. All the preparations were subjected to kinetic analysis by fitting the release data to different kinetic models to explain the release kinetics of ziprasidone from various preparations.
Procedure of drug release from rat abdominal skin
Rats weighing 135-160 gms were used to obtained freshly excised full thickness skin. Animals were sacrificed by spinal dislocation. Hairs from abdominal region was removed by means of surgical and razor taking care not to damage the epidermal surface, subcutaneous fat was removed carefully without damaging to skin. 700 mg of gel was spread uniformly on the epidermal surface and the skin was tied securely to the small beaker and the beaker was clamped in the organ bath to maintain the temperature. The drug release studies were carried out at time intervals of 15, 30, 45, 60, 90, 120, 150 and 180 min. The released drug was measured at 318 nm.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSION
The calibration curve of ziprasidone HCl was prepared in saline phosphate buffer pH 7.4 and the absorbance values of different concentrations of ziprasidone HCl in saline phosphate buffer pH 7.4 and absorbance were taken at 318 nm using UV spectrophotometer as shown in table 5 and fig 1
Table 5 Ziprasidone Hydrochloride absorbance at 318 nm
|
Concentration (µg/ml) |
Absorbance at 318 nm |
|
10 |
0.103 |
|
20 |
0.125 |
|
30 |
0.167 |
|
40 |
0.236 |
|
50 |
0.307 |
|
60 |
0.445 |
|
70 |
0.499 |
|
80 |
0.577 |
|
90 |
0.716 |
|
100 |
0.741 |
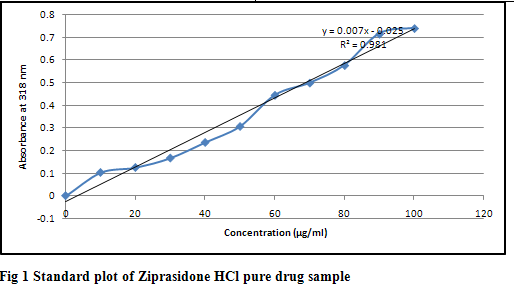
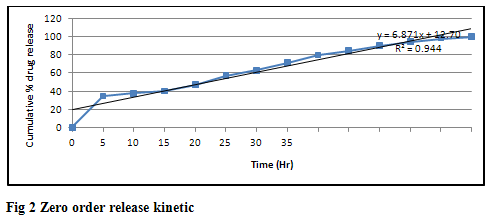
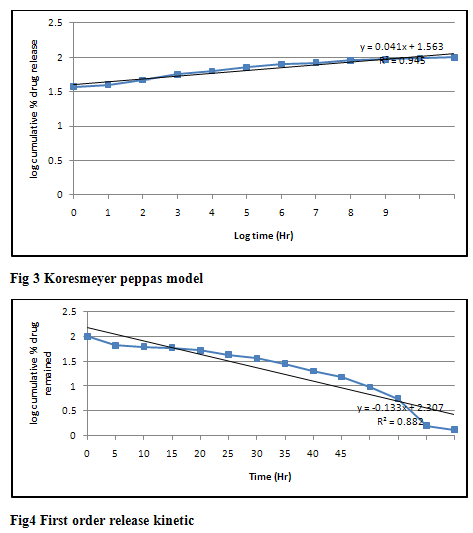
From in vitro drug dissolution studies we conclude that the formulations containing tulsi oil showed more release as compared to other enhancers and among four bases carbopol gel showed more release of drug as shown in table 6-18. The mechanism of action of tulsi oil is not well established yet but it might be possible that it modifies barrier properties to enhance the percutaneous absorption. In vitro drug release study through skin as shown in table 19 that the formulations containing tulsi oil releases the drug faster as compared to formulation G1 which does not contain tulsi oil through the skin. It may be concluded from the results that as the concentration of tulsi oil increases in the formulations the rate of drug also increases. It is clear that tulsi oil can significantly enhance the penetration of ziprasidone hydrochloride monohydrate from gel formulation across the skin. Kinetics result shown in table 20 of the optimized formulation G11 follows Higuchi kinetics as correlation coefficient (r2) value is higher than that of Zero order, First order and Koresemeyer peppas kinetics as shown in fig 2, 3 & 4. The calculated n value from power law equation for the formulation G11 was 0.041 indicating anomalous behaviour (also called as non-fickian diffusion) as a mechanism of drug release.
Table 6 In vitro release profile of formulations with and without enhancers
|
Time (hr) |
Without (G1-O1) Cumulative % age Release 5% DMSO(G2-O2) |
|||||||
|
G1 |
PG1 |
C1 |
O1 |
G2 |
PG2 |
C2 |
O2 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
8.23 |
7.97 |
7.97 |
7.58 |
17.10 |
15.68 |
14.40 |
13.50 |
|
1 |
9.00 |
8.35 |
8.23 |
7.84 |
19.67 |
17.74 |
17.10 |
15.55 |
|
2 |
10.15 |
9.90 |
9.13 |
8.87 |
21.08 |
20.15 |
19.48 |
17.71 |
|
3 |
10.41 |
10.41 |
9.64 |
9.13 |
25.88 |
24.24 |
23.80 |
21.74 |
|
4 |
12.47 |
10.67 |
10.67 |
10.15 |
29.95 |
27.17 |
26.85 |
23.41 |
|
5 |
14.78 |
12.98 |
11.18 |
10.67 |
33.94 |
31.15 |
30.04 |
28.57 |
|
6 |
15.43 |
14.53 |
11.57 |
11.31 |
36.67 |
34.08 |
33.64 |
31.37 |
|
7 |
16.33 |
14.78 |
12.21 |
11.57 |
40.10 |
39.62 |
38.82 |
35.81 |
|
8 |
16.71 |
14.91 |
12.73 |
11.95 |
44.41 |
42.71 |
41.37 |
40.74 |
|
9 |
16.58 |
15.17 |
12.86 |
12.08 |
48.37 |
46.90 |
45.32 |
45.44 |
|
10 |
18.00 |
15.43 |
13.11 |
12.34 |
53.32 |
50.08 |
49.51 |
48.88 |
|
11 |
18.77 |
15.56 |
13.24 |
12.34 |
59.41 |
56.88 |
52.31 |
52.20 |
|
12 |
19.80 |
15.81 |
13.88 |
12.60 |
64.85 |
61.45 |
59.91 |
57.37 |
Table 7 In vitro release profile of formulations with enhancers
|
Time (hr) |
10% DMSO (G3-O3) Cumulative % age Release 15% DMSO (G4 –O4) |
|||||||
|
G3 |
PG3 |
C3 |
O3 |
G4 |
PG4 |
C4 |
O4 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
18.38 |
16.84 |
15.81 |
14.66 |
20.70 |
18.51 |
16.58 |
15.56 |
|
1 |
20.44 |
19.41 |
17.22 |
16.20 |
25.33 |
20.06 |
19.03 |
18.39 |
|
2 |
24.81 |
22.60 |
20.77 |
19.38 |
29.96 |
24.69 |
21.99 |
20.44 |
|
3 |
27.12 |
25.07 |
23.60 |
22.32 |
33.95 |
28.67 |
24.81 |
23.76 |
|
4 |
30.08 |
28.80 |
27.94 |
25.37 |
37.16 |
31.63 |
28.67 |
26.84 |
|
5 |
36.27 |
32.27 |
30.02 |
28.31 |
41.66 |
38.85 |
31.24 |
29.52 |
|
6 |
40.81 |
36.55 |
34.99 |
33.64 |
45.39 |
42.80 |
33.95 |
32.44 |
|
7 |
43.51 |
40.22 |
39.66 |
36.09 |
49.38 |
46.89 |
36.13 |
35.27 |
|
8 |
49.27 |
45.54 |
42.11 |
40.99 |
52.08 |
50.85 |
40.38 |
39.82 |
|
9 |
54.10 |
51.50 |
48.77 |
46.30 |
57.74 |
55.39 |
43.30 |
41.87 |
|
10 |
58.31 |
55.22 |
53.28 |
51.26 |
65.45 |
63.12 |
46.16 |
47.99 |
|
11 |
62.52 |
60.15 |
59.59 |
57.73 |
73.68 |
70.88 |
52.53 |
51.29 |
|
12 |
69.51 |
66.62 |
63.68 |
62.10 |
79.86 |
74.68 |
61.23 |
59.16 |
Table 8 In vitro release profile of formulations with enhancers
|
Time (hr) |
20% DMSO(G5-O5) Cumulative % age Release 25% DMSO(G6-O6) |
|||||||
|
G5 |
PG5 |
C5 |
O5 |
G6 |
PG6 |
C6 |
O6 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
27.52 |
25.33 |
21.60 |
19.67 |
30.56 |
28.78 |
25.60 |
22.67 |
|
1 |
29.96 |
28.80 |
26.36 |
21.70 |
34.52 |
30.43 |
27.36 |
24.70 |
|
2 |
34.46 |
32.79 |
29.32 |
27.13 |
39.89 |
34.79 |
31.32 |
29.13 |
|
3 |
38.32 |
36.13 |
32.66 |
29.96 |
43.39 |
39.13 |
35.66 |
34.96 |
|
4 |
42.99 |
39.86 |
34.72 |
33.17 |
49.68 |
44.86 |
39.72 |
34.96 |
|
5 |
47.66 |
42.69 |
41.53 |
36.26 |
56.12 |
50.69 |
43.53 |
40.26 |
|
6 |
55.39 |
47.45 |
46.16 |
40.89 |
61.59 |
58.45 |
49.16 |
44.89 |
|
7 |
59.39 |
52.46 |
49.25 |
43.33 |
67.04 |
63.46 |
55.25 |
52.33 |
|
8 |
66.02 |
57.09 |
54.26 |
46.81 |
72.95 |
71.09 |
62.26 |
59.81 |
|
9 |
71.10 |
60.95 |
58.38 |
50.66 |
79.38 |
78.95 |
69.38 |
64.66 |
|
10 |
76.53 |
67.77 |
63.39 |
53.62 |
83.11 |
83.77 |
75.39 |
70.62 |
|
11 |
80.03 |
73.04 |
67.25 |
57.70 |
87.31 |
89.04 |
82.25 |
77.70 |
|
12 |
84.27 |
78.96 |
73.68 |
66.61 |
87.31 |
94.96 |
90.68 |
86.61 |
Table 9In vitro release profile of formulations with enhancers
|
Time (hr) |
5% tulsi oil (G7-O7) Cumulative % age Release 10% tulsi oil (G8-O8) |
|||||||
|
G7 |
PG7 |
C7 |
O7 |
G8 |
PG8 |
C8 |
O8 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
30.09 |
25.13 |
20.27 |
18.96 |
31.12 |
28.67 |
24.30 |
22.37 |
|
1 |
32.66 |
28.67 |
23.81 |
21.11 |
32.66 |
30.60 |
27.23 |
24.69 |
|
2 |
34.85 |
30.99 |
26.74 |
23.66 |
35.10 |
33.66 |
31.42 |
28.74 |
|
3 |
37.55 |
33.17 |
28.54 |
26.84 |
38.70 |
37.20 |
35.09 |
32.06 |
|
4 |
40.38 |
35.23 |
30.99 |
28.67 |
42.28 |
40.13 |
39.53 |
36.24 |
|
5 |
42.82 |
37.03 |
32.66 |
31.12 |
48.13 |
46.19 |
42.56 |
40.66 |
|
6 |
44.88 |
40.12 |
34.85 |
33.30 |
53.09 |
51.28 |
48.75 |
45.69 |
|
7 |
47.06 |
41.92 |
37.55 |
38.62 |
59.28 |
55.46 |
54.99 |
52.36 |
|
8 |
50.02 |
44.88 |
39.60 |
38.62 |
63.85 |
60.16 |
58.56 |
56.70 |
|
9 |
53.24 |
47.45 |
44.53 |
42.53 |
68.39 |
66.35 |
62.52 |
60.50 |
|
10 |
57.09 |
50.41 |
49.85 |
47.54 |
76.09 |
71.31 |
69.02 |
64.59 |
|
11 |
62.28 |
58.24 |
56.68 |
52.23 |
81.57 |
79.62 |
76.98 |
71.83 |
|
12 |
70.24 |
63.81 |
60.41 |
58.34 |
87.04 |
84.71 |
81.78 |
77.62 |
Table 10 In vitro release profile of formulations with enhancers
|
Time (hr) |
15% tulsi oil (G9-O9) Cumulative % age Release 20% tulsi oil (G10-O10) |
|||||||
|
G9 |
PG9 |
C9 |
O9 |
G10 |
PG10 |
C10 |
O10 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
32.53 |
30.22 |
26.23 |
23.53 |
33.43 |
31.50 |
28.03 |
24.17 |
|
1 |
34.82 |
32.02 |
28.03 |
25.20 |
36.13 |
33.82 |
30.60 |
26.10 |
|
2 |
38.13 |
35.82 |
32.96 |
28.90 |
39.35 |
36.26 |
33.69 |
29.54 |
|
3 |
42.60 |
39.87 |
36.27 |
32.60 |
44.95 |
40.45 |
36.52 |
32.02 |
|
4 |
47.56 |
43.96 |
40.46 |
38.53 |
52.42 |
46.28 |
40.48 |
38.85 |
|
5 |
51.52 |
49.28 |
45.55 |
43.46 |
61.25 |
52.98 |
45.56 |
42.19 |
|
6 |
59.25 |
54.10 |
51.60 |
49.26 |
68.98 |
61.29 |
51.26 |
47.40 |
|
7 |
63.82 |
59.19 |
56.30 |
54.96 |
73.32 |
68.76 |
57.19 |
51.23 |
|
8 |
68.88 |
63.66 |
61.23 |
60.28 |
81.38 |
77.59 |
66.63 |
59.68 |
|
9 |
74.32 |
71.85 |
68.16 |
66.13 |
86.69 |
82.91 |
73.62 |
64.76 |
|
10 |
79.25 |
76.52 |
72.53 |
71.38 |
90.42 |
86.09 |
79.55 |
71.98 |
|
11 |
84.98 |
80.61 |
79.75 |
76.11 |
93.25 |
91.41 |
87.48 |
78.39 |
|
12 |
89.10 |
86.57 |
82.22 |
80.81 |
97.70 |
94.11 |
90.92 |
84.84 |
Table 11 In vitro release profile of formulations with enhancers
|
Time (hr) |
25% tulsi oil (G11-O11) Cumulative % age Release 5% span (G12-O12) |
|||||||
|
G11 |
PG11 |
C11 |
O11 |
G12 |
PG12 |
C12 |
O12 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
34.85 |
35.66 |
30.22 |
26.62 |
10.67 |
08.12 |
07.48 |
07.22 |
|
1 |
37.67 |
37.46 |
32.40 |
28.54 |
11.31 |
10.64 |
08.38 |
08.04 |
|
2 |
40.38 |
40.01 |
34.85 |
32.37 |
13.34 |
10.64 |
10.02 |
08.04 |
|
3 |
46.98 |
43.89 |
39.52 |
37.69 |
16.37 |
13.82 |
12.05 |
10.56 |
|
4 |
57.19 |
47.33 |
43.32 |
41.87 |
19.40 |
16.72 |
15.08 |
13.57 |
|
5 |
63.13 |
52.26 |
50.95 |
48.19 |
23.81 |
19.14 |
18.24 |
16.60 |
|
6 |
71.39 |
61.96 |
57.63 |
56.53 |
29.22 |
22.55 |
21.01 |
20.50 |
|
7 |
79.84 |
66.67 |
63.66 |
60.99 |
32.77 |
28.22 |
26.68 |
24.65 |
|
8 |
84.67 |
72.98 |
70.72 |
64.42 |
39.31 |
33.51 |
31.10 |
29.55 |
|
9 |
90.34 |
81.04 |
79.52 |
71.25 |
46.62 |
39.15 |
36.14 |
34.84 |
|
10 |
94.32 |
86.35 |
84.74 |
78.24 |
52.58 |
44.47 |
42.18 |
40.51 |
|
11 |
98.41 |
91.68 |
89.69 |
85.61 |
57.38 |
50.17 |
49.01 |
47.67 |
|
12 |
100.24 |
95.81 |
93.04 |
91.69 |
62.82 |
57.12 |
54.30 |
51.60 |
Table 12 In vitro release profile of formulations with enhancers
|
Time (hr) |
10% span (G13-O13) Cumulative % age Release 15% span (G14-O14) |
|||||||
|
G13 |
PG13 |
C13 |
O13 |
G14 |
PG14 |
C14 |
O14 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
11.44 |
10.02 |
09.25 |
08.74 |
15.04 |
11.82 |
09.77 |
09.38 |
|
1 |
12.47 |
11.18 |
10.02 |
09.77 |
17.35 |
13.37 |
11.82 |
10.54 |
|
2 |
14.88 |
13.60 |
12.92 |
11.41 |
20.67 |
16.68 |
13.50 |
12.95 |
|
3 |
17.94 |
16.40 |
14.47 |
13.18 |
24.34 |
19.00 |
16.81 |
15.37 |
|
4 |
20.51 |
19.22 |
17.40 |
15.24 |
30.75 |
23.41 |
20.22 |
18.04 |
|
5 |
25.67 |
19.22 |
19.30 |
18.01 |
36.65 |
27.08 |
24.51 |
22.61 |
|
6 |
31.37 |
23.41 |
22.10 |
21.68 |
43.71 |
32.11 |
30.05 |
27.02 |
|
7 |
39.52 |
29.95 |
26.64 |
24.22 |
49.87 |
39.91 |
36.75 |
32.44 |
|
8 |
44.04 |
33.11 |
31.80 |
29.64 |
55.40 |
47.38 |
43.55 |
39.37 |
|
9 |
51.45 |
40.88 |
37.70 |
33.80 |
60.74 |
53.95 |
50.22 |
44.04 |
|
10 |
57.64 |
47.81 |
43.75 |
40.82 |
66.95 |
59.52 |
56.67 |
51.87 |
|
11 |
63.94 |
56.12 |
49.81 |
46.72 |
71.37 |
65.44 |
61.60 |
58.54 |
|
12 |
69.38 |
62.52 |
57.61 |
50.72 |
76.74 |
72.21 |
68.81 |
66.08 |
Table 13 In vitro release profile of formulations with enhancers
|
Time (hr) |
20% span (G15-O15) Cumulative % age Release 25% span (G16-O16) |
|||||||
|
G15 |
PG15 |
C15 |
O15 |
G16 |
PG16 |
C16 |
O16 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
16.32 |
13.75 |
11.44 |
10.41 |
18.12 |
15.68 |
13.24 |
11.44 |
|
1 |
17.87 |
15.81 |
13.37 |
12.08 |
19.15 |
17.61 |
15.04 |
13.37 |
|
2 |
19.67 |
18.25 |
15.17 |
14.75 |
21.08 |
20.90 |
18.22 |
16.78 |
|
3 |
22.34 |
20.18 |
17.61 |
16.68 |
24.88 |
23.08 |
21.67 |
19.10 |
|
4 |
25.27 |
24.60 |
21.80 |
19.48 |
28.68 |
27.88 |
25.34 |
23.51 |
|
5 |
29.45 |
28.65 |
26.70 |
24.15 |
33.48 |
31.94 |
29.14 |
28.80 |
|
6 |
33.51 |
32.32 |
30.88 |
29.95 |
39.41 |
36.35 |
33.22 |
31.21 |
|
7 |
38.98 |
32.32 |
33.94 |
32.88 |
43.47 |
41.67 |
39.54 |
36.52 |
|
8 |
46.94 |
40.38 |
38.84 |
37.81 |
49.94 |
46.72 |
44.21 |
41.32 |
|
9 |
52.38 |
47.31 |
44.90 |
42.87 |
56.18 |
52.55 |
50.88 |
47.87 |
|
10 |
59.60 |
53.01 |
51.72 |
48.41 |
63.65 |
59.10 |
56.55 |
53.67 |
|
11 |
66.54 |
60.81 |
57.55 |
53.37 |
74.31 |
64.54 |
61.61 |
59.88 |
|
12 |
79.01 |
68.87 |
64.38 |
61.78 |
84.32 |
76.88 |
68.67 |
64.58 |
Table 14 In vitro release profile of formulations with enhancers
|
Time (hr) |
5% oleic acid (G17-O17) Cumulative % age Release 10% oleic acid (G18-O18) |
|||||||
|
G17 |
PG17 |
C17 |
O17 |
G18 |
PG18 |
C18 |
O18 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
27.52 |
23.27 |
20.44 |
18.77 |
28.54 |
24.17 |
21.47 |
20.44 |
|
1 |
29.99 |
25.94 |
22.24 |
21.93 |
31.50 |
26.84 |
23.27 |
21.47 |
|
2 |
32.82 |
28.74 |
25.94 |
24.47 |
34.46 |
29.90 |
26.46 |
24.53 |
|
3 |
35.59 |
31.44 |
28.62 |
27.66 |
37.55 |
32.60 |
30.90 |
29.33 |
|
4 |
38.03 |
34.24 |
31.06 |
27.66 |
41.96 |
36.53 |
34.09 |
32.42 |
|
5 |
42.09 |
39.92 |
34.12 |
31.74 |
46.28 |
40.59 |
39.53 |
36.37 |
|
6 |
46.33 |
43.26 |
38.30 |
36.29 |
51.10 |
47.80 |
43.66 |
40.79 |
|
7 |
50.81 |
49.60 |
41.58 |
39.60 |
56.71 |
47.80 |
46.90 |
44.46 |
|
8 |
54.45 |
52.53 |
46.19 |
41.27 |
60.12 |
54.28 |
51.73 |
50.39 |
|
9 |
57.41 |
56.36 |
46.19 |
45.59 |
64.31 |
59.39 |
56.82 |
54.70 |
|
10 |
61.11 |
60.16 |
50.33 |
49.55 |
69.01 |
66.19 |
61.88 |
60.53 |
|
11 |
64.55 |
63.53 |
56.55 |
53.48 |
73.25 |
71.18 |
67.06 |
64.88 |
|
12 |
68.77 |
66.46 |
61.88 |
59.33 |
78.57 |
76.62 |
72.89 |
69.32 |
Table 15 In vitro release profile of formulations with enhancers
|
Time (hr) |
15% oleic acid (G19-O19) Cumulative % age Release 20% oleic acid (G20-O20) |
|||||||
|
G19 |
PG19 |
C19 |
O19 |
G20 |
PG20 |
C20 |
O20 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
29.82 |
27.13 |
24.17 |
21.34 |
31.24 |
29.70 |
26.74 |
24.17 |
|
1 |
32.27 |
29.67 |
26.59 |
23.66 |
33.30 |
32.37 |
28.67 |
26.10 |
|
2 |
35.23 |
32.99 |
29.52 |
25.84 |
37.13 |
35.82 |
31.12 |
29.42 |
|
3 |
38.19 |
37.46 |
32.96 |
28.74 |
40.67 |
38.85 |
34.30 |
32.60 |
|
4 |
43.53 |
37.46 |
36.53 |
31.67 |
44.95 |
38.85 |
37.87 |
36.53 |
|
5 |
49.85 |
42.48 |
39.46 |
35.37 |
50.73 |
46.89 |
41.96 |
40.69 |
|
6 |
53.81 |
49.53 |
43.77 |
41.82 |
55.09 |
51.85 |
46.28 |
44.75 |
|
7 |
61.73 |
54.10 |
49.86 |
45.87 |
61.02 |
57.68 |
51.59 |
49.70 |
|
8 |
67.53 |
60.81 |
52.66 |
50.19 |
68.11 |
64.99 |
59.52 |
55.25 |
|
9 |
72.24 |
66.35 |
59.10 |
57.38 |
74.39 |
70.92 |
66.68 |
61.92 |
|
10 |
78.55 |
71.31 |
66.16 |
61.56 |
79.12 |
75.52 |
71.99 |
68.10 |
|
11 |
78.55 |
74.39 |
71.53 |
66.78 |
84.57 |
80.71 |
77.31 |
72.42 |
|
12 |
81.42 |
79.87 |
75.14 |
71.73 |
86.39 |
84.28 |
82.42 |
79.63 |
Table 16 In vitro release profile of formulations with enhancers
|
Time (hr) |
25% oleic acid (G21-O21) Cumulative % age Release 5% PEG (G22-O22) |
|||||||
|
G21 |
PG21 |
C21 |
O21 |
G22 |
PG22 |
C22 |
O22 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
33.30 |
31.24 |
28.67 |
25.20 |
08.61 |
08.22 |
08.10 |
07.97 |
|
1 |
35.85 |
33.53 |
30.99 |
27.13 |
09.12 |
09.12 |
08.48 |
08.35 |
|
2 |
38.03 |
36.46 |
33.66 |
30.80 |
11.80 |
11.15 |
10.51 |
9.07 |
|
3 |
42.53 |
39.42 |
37.85 |
34.24 |
14.95 |
13.18 |
12.41 |
11.51 |
|
4 |
46.88 |
44.73 |
41.03 |
38.30 |
17.72 |
16.34 |
14.44 |
13.90 |
|
5 |
50.09 |
44.73 |
44.38 |
41.23 |
22.52 |
20.37 |
17.60 |
16.71 |
|
6 |
56.53 |
50.49 |
48.95 |
44.42 |
28.55 |
24.52 |
21.24 |
20.47 |
|
7 |
61.56 |
55.09 |
53.01 |
49.38 |
33.38 |
31.22 |
26.75 |
23.37 |
|
8 |
68.26 |
61.02 |
59.06 |
53.56 |
40.02 |
36.77 |
31.65 |
29.88 |
|
9 |
72.02 |
68.62 |
64.25 |
59.75 |
44.98 |
41.18 |
37.10 |
34.40 |
|
10 |
78.47 |
73.32 |
71.31 |
64.96 |
50.30 |
47.01 |
42.12 |
39.17 |
|
11 |
84.17 |
79.41 |
77.14 |
70.66 |
55.22 |
52.90 |
49.41 |
46.84 |
|
12 |
89.10 |
86.59 |
83.25 |
77.46 |
59.12 |
57.61 |
54.22 |
51.51 |
Table 17 In vitro release profile of formulations with and without enhancers
|
Time (hr) |
10% PEG (G23-O23) Cumulative % age Release 15% PEG (G24-O24) |
|||||||
|
G23 |
PG23 |
C23 |
O23 |
G24 |
PG24 |
C24 |
O24 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
10.41 |
09.12 |
08.48 |
07.22 |
14.65 |
10.80 |
09.25 |
08.61 |
|
1 |
11.95 |
10.67 |
09.90 |
08.45 |
16.97 |
12.60 |
11.18 |
09.54 |
|
2 |
14.01 |
12.47 |
11.67 |
11.02 |
19.97 |
15.04 |
13.24 |
11.44 |
|
3 |
17.68 |
15.40 |
14.08 |
13.41 |
24.70 |
18.84 |
16.18 |
13.11 |
|
4 |
20.61 |
19.94 |
18.14 |
16.31 |
28.24 |
22.38 |
19.58 |
16.65 |
|
5 |
26.15 |
24.10 |
23.55 |
20.31 |
33.27 |
28.67 |
23.61 |
20.98 |
|
6 |
30.98 |
29.70 |
28.45 |
25.14 |
40.32 |
34.70 |
29.77 |
26.12 |
|
7 |
34.52 |
29.70 |
32.38 |
30.55 |
46.74 |
40.24 |
35.31 |
31.67 |
|
8 |
39.60 |
34.95 |
36.15 |
34.22 |
51.88 |
47.65 |
41.11 |
36.08 |
|
9 |
43.55 |
40.27 |
40.31 |
39.25 |
55.84 |
53.58 |
47.30 |
42.88 |
|
10 |
51.38 |
46.81 |
44.62 |
42.15 |
61.67 |
60.02 |
53.61 |
49.05 |
|
11 |
56.30 |
52.61 |
49.17 |
47.80 |
69.60 |
66.98 |
60.95 |
55.51 |
|
12 |
62.12 |
59.24 |
54.22 |
50.95 |
73.30 |
70.81 |
67.27 |
62.67 |
Table 18 In vitro release profile of formulations with enhancers
|
Time (hr) |
20% PEG (G25-O25) Cumulative % age Release 25% PEG (G26-O26) |
|||||||
|
G25 |
PG25 |
C25 |
O25 |
G26 |
PG26 |
C26 |
O26 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.5 |
15.81 |
12.08 |
10.02 |
9.38 |
16.84 |
14.27 |
11.57 |
10.41 |
|
1 |
17.22 |
14.40 |
12.95 |
11.05 |
18.51 |
16.81 |
13.52 |
12.34 |
|
2 |
20.02 |
16.71 |
14.88 |
13.60 |
21.44 |
19.74 |
16.52 |
15.52 |
|
3 |
26.95 |
20.51 |
17.81 |
16.01 |
24.24 |
23.15 |
20.45 |
18.20 |
|
4 |
31.88 |
25.05 |
21.48 |
20.94 |
36.04 |
28.34 |
25.02 |
21.74 |
|
5 |
37.81 |
29.11 |
26.77 |
25.74 |
42.22 |
36.52 |
29.08 |
26.54 |
|
6 |
42.12 |
34.17 |
31.08 |
29.77 |
47.15 |
41.84 |
34.98 |
31.08 |
|
7 |
50.44 |
41.84 |
38.88 |
33.70 |
53.08 |
48.38 |
40.45 |
38.14 |
|
8 |
56.17 |
48.67 |
42.94 |
40.37 |
60.30 |
57.05 |
46.90 |
44.94 |
|
9 |
61.12 |
56.70 |
50.18 |
47.78 |
66.41 |
62.88 |
52.95 |
50.48 |
|
10 |
67.08 |
61.24 |
57.05 |
53.12 |
70.31 |
69.42 |
59.75 |
57.80 |
|
11 |
73.38 |
67.91 |
63.37 |
60.54 |
76.41 |
69.42 |
64.30 |
62.98 |
|
12 |
80.98 |
76.22 |
71.81 |
67.60 |
82.81 |
77.67 |
68.87 |
66.30 |
Table 19 In vitro drug release of carbopol gel through rat abdominal skin
|
Time (Min) |
Time2 |
%Drug release G1 |
% Drug release G7 |
%Drug release G8 |
% Drug release G9 |
%Drug release G10 |
%Drug release G11
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
15 |
3.872 |
3.85 |
4.20 |
5.32 |
8.37 |
10.13 |
15.32 |
|
30 |
5.477 |
6.08 |
8.37 |
10.41 |
11.20 |
13.36 |
16.63 |
|
45 |
6.708 |
13.26 |
13.82 |
14.37 |
18.19 |
24.58 |
26.45 |
|
60 |
7.745 |
18.13 |
20.17 |
22.12 |
28.36 |
33.72 |
38.63 |
|
90 |
9.486 |
21.15 |
23.23 |
25.78 |
37.10 |
39.88 |
42.26 |
|
120 |
10.954 |
28.67 |
29.91 |
31.72 |
41.36 |
43.91 |
46.52 |
|
150 |
12.247 |
33.61 |
34.43 |
36.32 |
42.49 |
44.53 |
48.87 |
|
180 |
13.416 |
33.80 |
36.53 |
39.18 |
42.73 |
45.76 |
49.81 |
Table 20 Correlation coefficient (R2) and release exponent (n) values for different kinetic models
|
Optimized formulation |
Zero order (R2) |
First order (R2) |
Koresmeyer peppas model (R2) |
n |
Higuchi (R2) |
|
G11 |
0.944 |
0.882 |
0.945 |
0.041 |
0.982 |
CONCLUSION
The permeability of ziprasidone hydrochloride was significantly enhanced by tulsi oil as a chemical penetration enhancer. Among five different enhancers tulsi oil is the best enhancer which showed more drug release as compared to other enhancer and among different bases gel formulation showed best results because the drug is hydrophobic in nature so the release order from different bases as follows: gel> proniosomal gel> cream> ointment.
REFERENCES
1. Lesem MD, Zajecka JM, Swift RH. Intramuscular ziprasidone, mg versus 10mg, in the short term management of agitated psychotic patients. J Clin Psychiatry 2001; 62.
2. Wilner KD, Hansen RA, Folger CJ, Geoffroy P. The pharmacokinetics of ziprasidone in healthy volunteers treated with cimetidine or antacid. Blackwell Science Ltd Br. J. Clin. Pharmacol 2000; 49: 57S-60S.
3. Aggarwal G, Dhawan S. Psychotropic drugs and transdermal drug delivery: an overview. International Journal of Pharma and Biosciences 2010; 2: 1-12.
4. Barry BW. Drug delivery routes in the skin: A novel approach. Adv. Drug Deliv. Rev 2002; 54: S31–S40.
5. Naik A, Kalia YN, Guy RH. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000; 3: 318–326.
6. Charoo NA, Shamsher AA, Kohli K, Pillai K, Rahman Z. Improvement in bioavailability of transdermally applied flurbiprofen using tulsi and turpentine oil. Colloids and Surfaces B: Biointerfaces 2008; 65: 300-307.
7. sarigullu OI, Yesim KH, Kantarci G, Sozer S, Guneri T, Ertan G. Transdermal delivery of Diclofenac sodium through rat skin from various formulations: AAPS PharmSci Tech 2006; 7(4): Article 88.
8. N. Mizobuchi, Y. Hasegawa, M. Kawada, et al. Stable aspirin-containing preparations for external use. In Remington 2001.
9. Sankar V, Ruckmani K, Durga S, Jailani S. Proniosomes as drug carriers. Pak. J. Pharm. Sci. 2010; 23(1): 103-107.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









