{ DOWNLOAD AS PDF }
ABOUT AUHTOR
Manish Patil*1, Harsha D Jani1, Suleman S Khoja2, Narmin A Pirani3, Shamim S Khoja3
1Department of Quality Assurance,
Shivam Pharmaceutical Studies and Research Centre, Anand
Gujarat, India.
2Resource person in pharmaceutical quality assurance and Audit Compliance, Vapi
3Registered Pharmacist, Gujarat, India
*manishpatil3194@gmail.com
ABSTRACT
This review article presents the pharmacology of combined Metformin hydrochloride and Teneligliptin hydrobromide hydrate is effective on type 2 Diabetes Mellitus. Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Teneligliptin, a third generation Dipeptidyl Peptidase-4 (DPP-4) inhibitor exhibits unique “J shaped” structure with “anchor-lock domain” mechanism which provides potent & long duration of action. The addition of teneligliptin once daily to Metformin was effective and generally well tolerated in Korean patients with type 2 diabetes. The mechanism of Metformin hydrochloride and teneligliptin hydrobromide hydrate is quite different. The main objective of this review article is to provide pharmacological and Analytical information of combination of Metformin hydrochloride and Teneligliptin hydrobromide hydrate to researcher in development of combined dosage form.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2470
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 3 Received On: 29/10/2016; Accepted On: 22/11/2016; Published On: 01/03/2017 How to cite this article: Patil M, Jani HD, Khoja SS, Pirani NA, Khoja S;A Review on chemistry and pharmacological activity of metformin hydrochloride and teneligliptin hydrobromide hydrate in combined dosage form; PharmaTutor; 2017; 5(3); 24-30 |
INTRODUCTION:
Type 2 diabetes mellitus:
It a medical condition characterized by an elevation of blood glucose level, this metabolic disorder will taken place as a result of either insulin resistance and/or insulin deficiency. This medical condition consider as one of the most predominant type of diabetes since it represent 90% of diabetic cases.
Moreover, this medical condition required a chronic monitoring and treatment throughout patient life; the treatment will involve several aspects like self-care measures, lifestyle changes (dietary modification) and in some cases medications (metformin and/or insulin). It has been observed during the past 50 years, the rate of incidence of this type of diabetes has markedly increased in parallel with obesity.
This medical problem i.e., high blood sugar when remain for long time this will mainly cause heart disease, strokes, diabetic retinopathy all these will lead to kidney damage.
Main Causes for its Incidence of Type 2 Diabetes Mellitus
Many factors play critical role in the incidence of this medical problem, like gender (female), age (increasing age), diet, obesity, lack of sleep, nutritional supplement received by the mother during pregnancy. But the main critical factors that play role are: lifestyle, genetic factors and other medical problems and also avoidance of medical checkup (periodically).
Lifestyle
This part will include many factors which are: obesity, stress, poor diet, high waist-hip ratio, high consumption of sweets (drinks and carbohydrate containing mainly glucose food) and lack of motivation and exercises.
Genetic
It has been found that several genes responsible for or related with incidence of type 2 diabetes, even so these genes still not consider as the main critical factor in incidence of this type of diabetes.
Other medical problems
This part include two subdivisions i.e., two factors associated with incidence of type 2 diabetes the first one include the use of some medications like [diuretics (thiazides), antihypertensive treatment (beta-blockers) and antipsychotic treatments. The second division include patients who sufferance from other diseases like gestational diabetes, Cushing’s syndrome, hyperthyroidism
Dipeptidyl Peptidase-4 (DPP-4) inhibitor
Dipeptidyl peptidase IV inhibitors are a class of oral anti hyperglycemic agents for the treatment of type 2 diabetes. The anti glycemic effect of DPP-4 inhibitors is mediated by inhibiting the degradation of the incretin hormone glucagon-like peptide-1 (GLP-1) and stimulating insulin release in response to increased blood glucose levels. Among all DPP-4 inhibitors, vildagliptin, saxagliptin and teneligliptin are peptide mimetic compounds, which have been discovered by replacing segments of peptide-based substrates. Whereas, sitagliptin, alogliptin and linagliptin are non-peptide mimetic compounds, which have been discovered by optimization of the initial lead compounds identified by random screening. Therefore, their chemical structures are diverse, suggesting that each of their binding modes in DPP-4 would be unique.
Metformin Hydrochloride
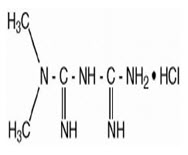
Metformin hydrochloride is an oral antihyperglycemic drug used in the management of type 2 diabetes. Metformin hydrochloride (Biguanide hypoglycemic agent)(N, N-dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5-HCL and a molecular weight of 165.63 g/mol. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether and chloroform. The pK, of metformin is 12.4.The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is as shown
PHARMACOKINETIC AND PHARMACODYNAMIC PROFILE (7)
Absorption and Bioavailability
The absolute bioavailability of a metformin hydrochloride 500 mg tablet given under fasting conditions is approximately 50-60%. Studies using single oral doses of metformin tablets of 500 mg and 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. Food decreases the extent of and slightly delays the absorption of metformin, as shown by approximately a 40% lower mean peak concentration (C,,,) and 25% lower area under the plasma concentration versus time curve (AUC), and a 35 minute prolongation of time to peak plasma concentration (Tmax) following administration of a single 850 mg tablet of metformin with food, compared to the same tablet strength administered fasting. The clinical relevance of these decreases is unknown.
Distribution
The apparent volume of distribution (V/F) of metformin following single oral doses of 850 mg averaged 654 + 358 L. Metformin is negligibly bound to plasma proteins in contrast to sulfonylureas which are more than 90% protein bound, Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin hydrochloride tablets, steady state plasma concentrations of metformin are reached within 24-48 hours and are generally c 1 pg/mL. During controlled clinical trials, maximum metformin plasma levels did not exceed 5 ug/mL, even at maximum doses
Metabolism and Elimination
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. Renal clearance (see Table 1) is approximately 3.5 times greater than creatinine clearance which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Teneligliptin Hydrobromide Hydrate:
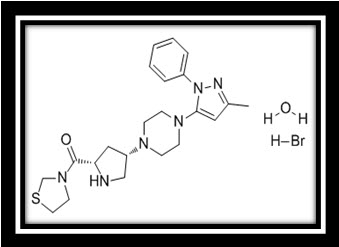
Teneligliptin, is chemically known as a {(2S,4S)-4-[4-(3-Methyl-1-phenyl-1H-pyrazol-5-yl)-1-piperazinyl]-2-pyrrolidinyl}(1,3 thiazolidin-3-yl) methanone hemipentahydrobromide hydrate thiazolidine hemipentahydrobromide hydrate and is peptidomimetic with the molecular formula of C22H30N6OS.2½HBr.xH2O and molecular weight of 628.86 g/mol g/mol for hemipentahydrobromide. The hydrate can be from mono to dihydrate.
Photostability:
Long term: 25oC / 60o RH, 8 month (30 month at RT)
Accelerated Stability Studies 40oC /175o RH
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Pharmacokinetics
Plasma concentration
1. Single – dose administration:
The plasma concentration changes and the pharmacokinetic parameters of teneligliptin after a single oral dose of 20 mg and 40 mg of teneligliptin given empty stomach to the healthy adults are shown below.
Table 1. Pharmacokinetic parameters at the time of single dose oral drug administration in healthy adults
|
Strength |
Cmax (ng/ml) |
AUC0-inf (ng.hr/ml) |
tmax (hr) |
T1/2 (hr) |
|---|---|---|---|---|
|
20mg |
187.20 ±44.70 |
2028.9±459.5 |
1.8 (1.0-2.0) |
24.2±5.0 |
|
40mg |
382.40±89.83 |
3705.0±787.0 |
1.0 (0.5-3.0) |
20.8±3.2 |
n=6, Mean Value±SD, tmax=Central value (minimum value -maximum value)
2. Repeated – dose administration: The pharmacokinetic parameters of teneligliptin after a repeated dose of 20 mg of teneligliptin once daily for seven days given 30 minutes before breakfast to the healthy adults are shown below. The state of equilibrium will be attained within seven days.
Table 2. Pharmacokinetic parameters at the time of repeated dose oral drug administration in healthy adults
|
|
Cmax (ng/ml) |
AUC0-24 (ng.hr/ml) |
AUC0-inf (ng.hr/ml) |
tmax (hr) |
T1/2 (hr) |
|---|---|---|---|---|---|
|
After first dose |
160.60± 47.26 |
1057.2± 283.9 |
1627.9± 427.8 |
1.0(0.4-2.0) |
25.8± 4.9 |
|
7 days after administrat-ion |
220.14± 59.86 |
1514.6± 370.5 |
2641.4± 594.7 |
1.0(1.0-1.0) |
30.2± 6.9 |
n=7, Mean Value±SD, tmax=Central value (minimum value - maximum value)
3. Food effect: Cmax decreased after a single dose of 20 mg of teneligliptin given post meal to the healthy adults as compared to empty stomach and tmax prolonged from 1.1 hr to 2.6 hr; however, no difference observed in AUC
Table 3. Pharmacokinetic parameters at the time of fasting and after food intake in healthy adults
|
|
Cmax (ng/ml) |
AUC0-72 (ng.hr/ml) |
AUC0-inf (ng.hr/ml) |
tmax (hr) |
T1/2 (hr) |
|---|---|---|---|---|---|
|
Empty Stomach |
232.2 (236.2 ±43.77) |
1855.5 (1861.1± 148.1) |
2090.3 (2094.6 ±138.5) |
1.1±0.4 26.5 |
26.5 (27.8±9.3) |
|
Post meal |
184.9 (187.5 ±33.55) |
1806.0 (1814.6 ±183.3) |
2044.0 (2056.1 230.9) |
2.6±1.1 26.9 |
26.9 (28.3±9.5) |
n=14, Geometric mean (Arithmetic mean value±Standard Deviation) tmax=Arithmetic mean value±Standard Deviation
Rate of protein binding
The protein binding ratio was 77.6%-82.2% when the label teneligliptin (20, 100, and 500 ng/ml) was added to the human plasma (in vitro)
Metabolism: Following a single oral administration the 20mg label teneligliptin to the healthy adults, the unaltered substance and the metabolites M1, M2, M3, M4, and M5 were observed in the blood plasma. Furthermore, the ratio of AUC of teneligliptin, M1, M2, M3, M4, and M5 with respect to AUC calculated from the plasma radioactive concentration up to 72 hours after administration was 71.1%, 14.7%, 1.3%, 1.3%, 0.3%, and 1.1%. Mainly CYP3A4 flavin containing monooxygenases participate in the metabolism of teneligliptin. Furthermore, although it showed a weak inhibitory action towards CYP2D6, CYP3A4, and FMO (IC50 value: 489.4, 197.5, and 467.2μmol/L), it did not showed inhibitory action towards CYP1A2, CYP2A6,CYP2B6, CYP2C8, CYP2C8/9, CYP2C19, and CYP2E1, and CY P1A2 and CYP3A4 were not introduced (in vitro).
Excretion:
1. When a single oral dose of 20 mg and 40 mg teneligliptin was given to the healthy adults on empty stomach, about 21.0 to 22.1% of dose was excreted as unaltered substance in urine, and the renal clearance was 37 to 39 mL/hr/kg.
2. When a single 20mg dose given to healthy adults, 45.4% of dosage radioactivity was excreted in urine and 46.5% was excreted in faeces up to 216 hours after administration. Furthermore, with respect to the dosage up to 120 hours after administration, the accumulated urinary excretion rate of unaltered substance, M1,M2, and M3 was 14.8%, 17.7%, 1.4%, and 1.9%, respectively and the accumulated faeces excretion rate of unaltered substance, M1, M3, M4, and M5 was 26.1%, 4.0%, 1.6%,0.3%, and 1.3%, respectively.
3.Teneligliptin is a substrate of P-glycoprotein that inhibited the transportation of digoxin up to 42.5% through P-glycoprotein in the concentration of 99μmol/L. Furthermore, it showed a weak inhibitory action towards the organic anion transporter OAT 3 appeared in kidney, (IC50 value: 99.2μmol/L); however, it did not showed inhibitory action towards OAT 1 and organic cation transporter OCT2 (in vitro).
Pharmacodynamics
DPP-4 INHIBITORY ACTION AND GLP-1 DEGREDATION INHIBITORY ACTION
1. Teneligliptin inhibits concentration-dependent human plasma DPP 4 activity, and its IC50 value (95% CI) was 1.75 (1.62 - 1.89) nmol/L (in vitro).
2. Teneligliptin concentration-dependently suppressed the degradation of GLP-1 in rat plasma, with IC50 values and its 95% Cl beings 292nM.
3. In the glucose tolerance test using Zucker Fatty rat, an obesity model showing insulin resistance and abnormal glucose tolerance, teneligliptin increased plasma active form GLP-1 concentration and plasma insulin concentration by its single-dose administration.
4. In patients having type 2 diabetes mellitus, the administration of 20 mg teneligliptin once daily inhibited the plasma DPP-4 activity and increased the plasma active form GLP-1 concentration
GLUCOSE TOLERENCE IMPROVEMENT ACTION
1. In the glucose tolerance test using Zucker Fatty rat, an obesity model showing insulin resistance and abnormal glucose tolerance, teneligliptin controlled an increase in the blood sugar level by its single-dose administration
2. In patients having type 2 diabetes mellitus, the administration of 20 mg teneligliptin once daily improved the blood sugar after breakfast, lunch, and dinner and the fasting blood sugar.
Combination Therapy of Metformin Hydrochloride and Teneligliptin Hydrobromide Hydrate
A total of 204 patients with type 2 diabetes participated in a 16-week, randomized, double-blind, placebo controlled study design to assess the efficiency of teneligliptin in combination with metformin. Patients with type 2 diabetes were eligible to participate if they had inadequate glycemic control ((HbA1c)levels 7.0-10.0% on stable dose of metformin monotherapy (>1000mg/day) for at least 8weeks). After the 2-week run-in period, eligible patients were assigned 2:1 to a 20mg teneligliptin once daily (N=136) or a placebo once daily (N=68) group, respectively. The metformin dose was kept constant throughout the study period. In combination with metformin, teneligliptin provide significant improvement in A1C and 2 hr PPF compared to placebo with metformin.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MECHANISM OF ACTION
Metformin
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia in either patients with type 2 diabetes or normal subjects and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
Teneligliptin
The glucagon-like peptide-1 (GLP-1) is secreted from alimentary canal in response to meal that promotes insulin secretion from pancreas and regulates blood sugar post meal by controlling glucagon secretion. Teneligliptin exhibits a hypoglycemic effect by controlling the degradation of GLP 1 by inhibiting dipeptidyl peptidase-4 (DPP-4) activity and thereby increasing blood concentration of active GLP-1.
Drug Profile:
|
Sr. No. |
Parameter |
Description |
|
|---|---|---|---|
|
Name of Drug |
Metformin Hydrochloride |
Teneligliptin Hydrobromide Hydrate |
|
|
1 |
Chemical Structure |
|
|
|
2 |
IUPAC Names |
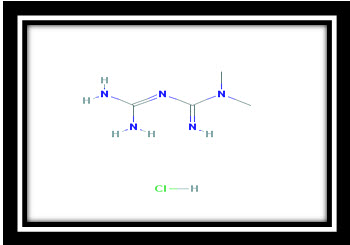
1-carbamimidamido-N,N-dimethylmethanimidamide |
{(2S,4S)-4-[4-(3-Methyl-1-phenyl-1H-pyrazol-5-yl)-1-piperazinyl]-2-pyrrolidinyl}(1,3-thiazolidin-3-yl) methanone hemipentahydrobromide hydrate |
|
3 |
Chemical Formula |
C4H11N5. HCL |
C22H30N6OS. 2.5 HBr. H2O |
|
4 |
Molecular Weight |
129.16 g/mol |
628.86 g/mol |
|
5 |
Appearance |
White powered |
White to Light Tan Solid |
|
6 |
Solubility |
Freely soluble as HCl salt in water |
DMSO, Methanol, Water |
|
7 |
Melting Range |
223-226 °C |
188-190 °C |
|
8 |
Therapeutic Category |
Hypoglycemic Agent (Biguanide) |
Hypoglycemic Agent (DPP IV inhibitor) |
|
9 |
Pka |
12.4 |
8.7 |
|
10 |
Log P |
-0.5 |
2.59 |
|
11 |
Storage |
Room Temperature |
Refrigerator |
2.2 Available Brands in market
|
Brand Name |
Content |
Company Name |
|---|---|---|
|
Zita Met Plus 20/500 Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Glenmark |
|
Totaglipt 10 tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Mankind Pharma |
|
Afoglip M 500 Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Torrent Pharma |
|
Glytrin Met 20/500tmg Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Medley Pharma |
|
Teniva 10 Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Intas |
|
Ziten M 20/500mg Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Glenmark |
|
Tendia M 500mg Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Eris Life sciences |
|
Tenglyn M 500mg Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Zydus Cadila |
|
Eternex M 500mg Tablet 10s |
Teneligliptin hydrobromide hydrate-20mg Metformin HCl-500mg |
Alembic Pharma |
CONCLUSION: By reviewing the all literatures, the combination therapy was found to be effective in treatment of Diabetes mellitus. This review represents individual pharmacology and pharmacokinetic of Metformin hydrochloride and Teneligliptin hydrobromide hydrate as well as mechanism of action of combination of Metformin hydrochloride and Teneligliptin hydrobromide hydrate in treatment of diabetes mellitus. This review will helpful for researcher in future study and also for development of combined formulation.
REFERENCES:
1. Bassam Abdul Rasool Hassan “Overview on Diabetes Mellitus (Type 2) Clinical Pharmacy Discipline”, School of Pharmaceutical Sciences, University of Sains Malaysia, 11800, Minden, Penang, Malaysia, 1993
2. Manish Maladkar, Srividya Sankar, Kushal Kamat “Teneligliptin: Heralding Change in Type 2 Diabetes” Aristo Pharmaceuticals Pvt. Ltd., Mumbai, India, 2016
3. M. Tamilselvi, A. Srinivas “Bioequivalence and Pharmacokinetics of Metformin Hydrochloride 1000 mg Tablet Extended Formulation in Healthy Indian Male Volunteers” journal of pharmaceutical and bioscience, 2014
4. Mitsubishi Tanabe Pharma, Corporation Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Report on the Deliberation Results, 201
5. Dr. Monica Bhatia, Bhagyalakshmi N, Kavitha M T, Arun Kharkwal, Rashmi Suresh, Ashutosh Mishra, Lim Kah Heng, Oon Chien Hsiang, Ashwini Lokhande, UBM Medica India Pvt. Ltd.
6. Metformin HCl drug profile, http://www.drugbank.ca/drugs/DB00331
7. Metformin HCl drug profile, http://www.rxlist.com/fortamet-drug.html
8.Teneligliptin hydrobromide hydrate Drug Profile, http://www.pharmacodia.com/web/drug/1_241.html
9. Teneligliptin hydrobromide hydrate Drug Profile, https://en.wikipedia.org/wiki/Teneligliptin ,
http://www.trc-canada.com/product-detail/?T0183000
10. Teneligliptin hydrobromide hydrate Drug Profile, http://www.genome.jp/dbget-bin/www.bget?dr:D09756
11.AvailableBrands in Market of this combination http://www.sastimedicine.com/salt-alternatives/13663-2142205/Teneligliptin-20mg-2B-Metformin-500mg-Price-Dosage-Side-Effects-and-Generic Alternatives
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE