ABOUT AUTHORS:
Vivek P. Chavda*, Maunit B. Mehta, Hamir B. Vala, Nirav A. Pandya
Department of Pharmaceutics, B.K. Mody Government Pharmacy College,
Rajkot – 360003, Gujarat (India)
*vivek7chavda@gmail.com
INTRODUCTION
Early 19th century, Mathes developed the first capsule dosage form from gelatin. We are familiar with capsule since a century.[1] It has been transformed in a different way to serve the society and to provide the additional benefits rather than the conventional gelatin capsule. A capsule is a shell or a container prepared from gelatin containing one or more medicinal and/or inert substances.[2] The gelatin capsule shell may be soft or hard depending on their formulations. Capsules are solid dosage forms in which the medication contained within gelatin shells. The medication may be a powder, a liquid or a semisolid mass. Capsules are intended to be swallowed whole by the patient. In instances where patients (especially children) are unable to swallow capsules, the contents of the capsule can be removed and added (e.g., sprinkled) on soft food immediately before ingestion. In this case, capsules are used as a vehicle to deliver premeasured medicinal powder. Capsule dosage forms occupy more than 10% of the total dosage forms on the market.[3] Soft gelatin capsule are available in oblong, spherical, elliptical and other shapes. They are also available in sizes to contain from 0.1 to 30 ml of volume. The various size of hard gelatin capsule is given in table 1.
Reference ID: PHARMATUTOR-ART-1951
Table 1: Size of hard gelatin capsule and its capacity
|
Size |
000 |
00 |
0 |
1 |
2 |
3 |
4 |
5 |
|
Volume (cm3) |
1.37 |
0.95 |
0.68 |
0.5 |
0.37 |
0.3 |
0.21 |
0.13 |
Advantages of Capsule [3-8]
- They may be used to mask the unpleasant tastes, aromas, or appearance of a drug
- Products can be encapsulated in various shapes, sizes and colors
- Products with thick slurry type paste medicaments to light oils and powders (with inert media) can be encapsulated in soft capsule
- They offer the pharmacist versatility to prepare any dose desired for a variety of administration routes (e.g. oral, inhalation, rectal, or to be diluted for vaginal, rectal, oral or topical use)
- Lower dose of active ingredients and given as unit dose
- High accuracy in fill weights
- Improved stability
- Longer shelf life
- Improved bioavailability
- They can be colored to protect the content from light and improve the acceptability
- Required less excipient than tablets.
Disadvantages [3, 5, 7, 8]
- They are easily tampered
- They are subject to the effects of relative humidity and to microbial contamination.
- More expensive (commercially)
- Bulk dosage cannot be dispensed in capsule
- Additional quality control measures may be required
Composition of Capsule Shell [6, 7]
Gelatin (See Table 2) is the most important constituent of the dipping solutions, but other components are also present to impart desired charecteristics.
1.Gelatin
2. Plasticizer: glycerin, sorbitol
3. Water
4. Preservatives: Methyl Paraben, Propyl Paraben
5. Colorants: F.D & C, Certified lakes
6. Opacifier: Titanium dioxide
7. Flavoring agent: Ethyl vanillin
8. Fumaric acid is added to aid solubility and to reduce aldehydes tanning of gelatin
Table 2: Gelatin Specifications for capsule shell[8]
|
Gelatin properties |
Hard shell |
Soft shell |
|
Bloom Strength (gm) |
2220-260 |
160-210 |
|
Viscosity |
3.5-4.5 |
3-3.5 |
|
Moisture (%max) |
13 |
13 |
|
Ash (%max) |
1 |
1 |
|
PH ,1% solution |
5.5-6 |
5-6 |
|
Iso ionic point |
7-9.4 |
7-9.4 |
|
Particle size % passing 4 us # |
100 |
100 |
|
SO2 (ppm max) |
40 |
60 |
|
Microbial Total count |
500 |
500 |
|
E.coli in 10 gm |
Absent |
Absent |
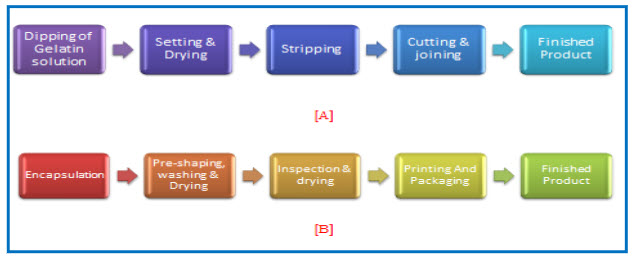
Figure 1: Manufacturing Steps for hard & soft gelatin Capsule
Novel technologies for capsule system
1. Novel Ring type of capsule
Mouzam I et al. (2011) have prepared novel floating ring capsule-type dosage form for stomach specific delivery.Study objectives were to develop a unique floating ring capsule dosage form which combines gastric soluble and insoluble portions, and to evaluate its suitability for stomach specific drug delivery. New floating ring capsules were developed using different polymers and were compared for various parameters. The formulation with HPMC and sodium CMC has better floating properties. The effects of polymers concentration on drug release were studies by in vitro release studies. The interaction studies of combined drug with polymers were determined using FT-IR spectroscopy. The entrapped air within the gel barrier and lower densities of HPMC and sodium CMC resulted in better floating behavior. Steady slow gel formations showed prolonged drug release. The in vitro release rates were generally found to be faster with low concentration of carbopol showing release within 2 h, while formulations containing high amount of HPMC showed release in 8 h. In particular, the higher concentration of HPMC formulation shows the best drug release performance. A very low change in peak shift was observed only with sodium alginate formulations. Further, FT-IR measurements confirmed the absence of any chemical interactions.[9]
2. Capsule with asymmetric membrane
Osmotically regulated asymmetric capsular systems prepared for simultaneous sustained delivery of various drug deliveries. The system would provide release of two or more drugs, which is helpful in reducing problems associated with multi drug treatment. In addition system also provide near zero order release which is require for controlled drug release. Asymmetric membrane capsule is prepared by using a phase inversion process in which the membrane structure is precipated out on a stainless steel mold pin by dipping the mold pin in coating solution comprised of 15% w/v of CA and various proportions of glycerol dissolved in ethanol and acetone and air dried for 15 sec. followed by quenching in aqueous solution for 3 min. which allowed formation of asymmetric membrane. After preparation of asymmetric membrane capsule is dried for about 12 hr at ambient temperature.[10]Prabhakaran et al. developed asymmetric capsular system for sustained delivery of Rifampicin and Isoniazide for treatment of tuberculosis. [11]Anil Philip and kamla pathak developed asymmetric membrane for controlled delivery of poorly soluble drug ketoprofen. Asymmetric membrane capsule was made by dry method via precipitation of asymmetric membrane on the walls of hard gelatin capsule. Resulting asymmetric membrane composed of a dense outer region with fewer pores and a lighter inner porous region.[12] Guan et al developed a Novel Gastro-Retentive Osmotic Pump Capsule Using Asymmetric Membrane Technology.[13]Garg et al developed asymmetric membrane capsule for antihypertensive drugs Atenolol and Amlodipine Besylate using wet phase inversion process in which glass pins were dipped into polymer solution containing cellulose acetate dissolved in mixture of acetone,alcohol and sorbitol, followed by quenching in a 10% v/v aqueous solution of sorbitol for 10 min. and then air dried and finnaly trimmed. Here, compartment prepared by first mixing Atenolol KCL and citric monohydrate in polyethylene bag then filled in capsule and Amlodipine Besylate ,KCL and citric monohydrate filled manually in capsule cap then both sealed and coated. [14]
3. Telemetric Capsule
The Telemetric capsule contains a location detector, transmitter, lithium battery, and interchangeable drug reservoir tip . As a consequence, it is the bulkiest of the devices at 39 mm in length and weighing 3.5 g. With the tracking cogwheel exposed, the capsule expands to a width of 20 mm, which presumably explains reports of prolonged stomach retention. Unfortunately, the activation mechanism has a high current drain limiting the device to only 8 h of operation, although this could be extended with developments in battery technology. Activation is triggered by an external magnet, which operates a magnetic switch within the capsule to connect the battery to a microfurnance. This, in turn, breaks a plastic strip, which releases a previously compressed spring to clear the aspiration orifice. The drug reservoir is under vacuum and so the capsule contents, in the form of a solution, are immediately forced out. Again with this device, particulate delivery is made difficult by the narrow release orifice.[15]
4. CODAS technology (Chronotherapeutic oral drug absorption system)[16, 17]
In certain cases, immediate release of drug is undesirable. A delay of drug action may be required for a variety of reasons. Chronotherapy is an example of when drug release may be programmed to occur after a prolonged interval following administration. Elan Drug Technology developed CODAS® technology to achieve this prolonged interval. The many advantages of the CODAS® technology include a delivery profile designed to complement circadian pattern, controlled onset, an extended release delivery system, rate of release essentially independent of pH, posture and food, “sprinkle” dosing by opening the capsule and sprinkling the contents on food, reduction in effective daily dose and drug exposure, gastrointestinal tract targeting for local effect and reduced systemic exposure to achieve a target profile.Verelan® PM uses the proprietary CODASTM technology, which is designed for bedtime dosing, incorporating a 4- to 5-h delay in drug delivery. The controlled-onset delivery system results in a maximum plasma concentration (Cmax) of verapamil in the morning hours.
5. Innercap Technology
The Fig. 2 shows how four individual compounds are combined into one single dosage form. The combination example consists of a high potency insoluble active in a lipid emulsion, sustained release tablet and a cocktail of two crystalline active materials. Acombination of release profiles can be incorporated in the system.
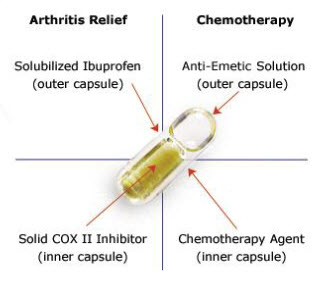
Figure 2: Innercap technology( innercap.com)
6. Port Capsule Technology
Port stands for programmable oral release technologies that use a unique coated in capsulated system with opportunity to provide multiple program release of drug. Port technologies offer significant flexibility in obtaining unique and desirable release profile to maximize pharmacological and therapeutic effect. There are mainly two dosage forms for port technology. The dosage from consist of a hard gelatin capsule coated with the semi permeable, rate controlling polymer. Inside coated capsule is the osmotic energy source, which normally contains the therapeutic agents to be delivers. The capsule is sealed with the water in soluble lipid separators plug and immediate release dosage can be edit above the plug the to complete the dosing option.[18]
7. CHEWCAPS™
Encap Drug Delivery a division of capsugel has explored various formulations for “chewable” capsules containing a range of flavors. The mouthfeel is agreeable as the capsule body is crushed and folded into the flavored matrix by chewing. Gelatin capsules simply fracture, creating a very uncomfortable sensation. Here, the permeability of the HPMC product is an advantage. Typically, gelatin capsules do not transmit odor. For chewable, where taste is significant to user acceptability, the perceived flavor may enhanced by perception of product odor on opening the (bulk) pack.
8. Fast disintegrating capsule for oral cavity[19]
Fast disintegrating capsules were prepared by either by perforation or by vaccum-drying of conventional hard gelatin capsules. Preparation of fast disintigrating capsules were done by perforating with needle at the top or along the longitudinal section to reduce the disintegration time of capsule and other method is vaccumdrying of the prepared capsules. Varioustechnologies for orally disintegrating dosage forms have been developed , such as lyophilized formulations, fast-dissolving tablets and oral films. The ZydisTM dosage form, developed by Scherer is the fastest dissolving system available and dissolves within seconds in the oral cavity.
9. Duo caps TM
It is more specially grafted for pulsatile delivery which also solves the compatibility issue with improved patient compliance. Their preparation is often complex at bench level and proportionately difficult at manufacturing scale. Using its liquid fill technology for hard capsules, Drug Delivery has developed a practicable and convenient formulation system that is well suited to such multiphase products (DuoCap™). It has also designed and built machine modules that enable full- scale manufacturing on Bosch-based equipment. It enables a capsule (wide range of formats, including coated) to be filled into a larger liquid-filled capsule, which may, in turn, be coated.[20]
REFERENCES
[1] Vilivalam VD, Kurshid I. Starch capsules: an alternative system for oral drug delivery. PSTT 2000;3:63-9.
[2] Chavda VP, Parmar RB, Soniwala MM, Chavda JR. Vegitarian capsule Mintage journal of Pharmaceutical and medical sciences 2013:Accepted.
[3] Banker SG, Rhodes T. A Hand Book Of Modern Pharmaceutics. Fourth Edition Revised And Expand Published And Distributed By Marcel Dekker New York, 2011:335-75.
[4] Loyed V. Allen Jr, Nicholas G. Popovich, Howard C. Ansel. Pharmaceutical Dosage Forms And Drug Delivery System, 8th Edition, Publishers and Distributors by Lippincott Williams And Wilkins, USA, 204-226.
[5] Micheal E. Aulton. A Hand Book Of Aulton?s Pharmaceutics (The Design And Manufacture Of Medicines), Chapter 34 (Hard Gelatin Capsule) & Chapter 35 (Soft Gelatin Capsule), Third Editon, Published And Distributed By Churchill Livingstone Elsevier Philadelphia And Printed In Hungry, 515-538.
[6] Reich G. The Text Book Of Pharmaceutical Capsules”, Second Edition, Edited By Fridrun Podczek And Brian E Jones And Published By Pharmaceutical Press Great Britain By Antony Rowe Ltd., Chippenham 2004, Available On- pharmpress. com/ files/ docs/chap%2011.pdf.
[7] Leon Lachman, Herbert A. Liberman, Joseph L. Kanig . The Theory And Practice Of Industrial Pharmacy. Third Edition, Published And Distributed By Varghese Publishing House, Dadar, Bombay-400014, 374-412.
[8] Remington?s: The Science And Practice Of Pharmacy, Volume-I, 20th Edition, Publishers And Distributors By Lippincott Williams And Wilkins, 885-890.
[9] Mouzam I, Dehghan MG, Asif S. Preparation of a novel floating ring capsule-type dosage form for stomach specific delivery. Saudi Pharmaceutical Journal 2011;19:85-93.
[10] D. Prabakaran PS, K.S. Jaganathan, Suresh P. Vyas. Osmotically regulated asymmetric capsular systems for simultaneous sustained delivery of anti-tubercular drugs. Journal of controlled release 2003;94:239-48.
[11] Anil philip KP. In Situ-Formed Asymmetric Membrane Capsule for Osmotic Release of Poorly Water-Soluble Drug. PDA Journal of Pharmaceutical Science and Technology 2007;61:24-36.
[12] Jin Guan LZ, Yusheng Pan,Haitao Han,Hongtao Xu,Weisan Pan A Novel Gastro-Retentive Osmotic Pump Capsule Using Asymmetric Membrane Technology: In Vitro and In Vivo Evaluation. 2010;27:105 14.
[13] Saurabh GARG KP, Anil PHILIP, Dinesh PURI. Osmotically Regulated Two-Compartment Asymmetric Membrane Capsules for Simultaneous Controlled Release of Anti-Hypertensive Drugs. scientica pharmaceutica 2011;80:229-50.
[14] Pratim K. Choudhury MSR, Mousumi K. Pillai,Chetan S. Chauhan. Asymmetric membrane capsule for osmotic delivery of flurbiprofen. Acta pharma 2007;57:343-50.
[15] Wilding I, Connor A. Development of a new engineering-based capsule for human drug absorption studies. Pharmaceutical Science & Technology Today 2000;3:385–92.
[16] Celene M. Amabile Bill, Bowman J.Oral modified-release opioid productsfor chronic pain management: oral modified release opioid products;The Annals of Pharmacotherapy. 2006;40(7):1327-35.
[17] Prajapati BG & Solanki H. Recenttechniques for oral time controlled pulsatile technology.The internet journalof third world medicine,2009; 8(1).
[18] Parmar RD, Parikh RK, Vidyasagar G, Patel DV, Patel CJ, Patel BD. Pulsatile Drug Delivery System: An Overview. Int J Pharma Sci Nanotechno 2009;2:605-14.
[19] Mesut Ciper RB. Modified conventional hard gelatin capsules as fast disintegrating dosage form in the oral cavity. European Journal of Pharmaceutics and Biopharmaceutics 2006;62:178-84.
[20] William Bowtle. Advances In Liquid Filled Capsule Technology” Published In Business Briefing Pharmatech, 1-4.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









