 ABOUT AUTHORS:
ABOUT AUTHORS:
Jalaram H Thakkar*, Ashiyana A Mansuri, Chirag Patel, Girish K Jani,
SSR College of Pharmacy,
Silvassa.
*jay_143143@yahoo.com
{ DOWNLOAD AS PDF }
ABSTRACT:
The term Angiogenesis was coined by DR. John Hunter, a British surgeon in 1787. Later professor Judah Folkman gave the idea that growth of tumor depends on their blood supply and a new began research in this new field of therapeutics called ANGIOGENESIS. Then, the identification of angiogenic growth factors, Vascular Endothelial Growth Factor (VEGF) and Fibroblast Growth Factor (FGF) fueled interest in using these factors in inducing therapeutic angiogenesis.[1] Initially angiogenesis was implicated only in few diseases like cancer, psoriases and arthritis. But, in recent years, it has been evident through a deep research that even excessive, insufficient and abnormal angiogenesis contribute to the pathogenesis of many disorders. Angiogenesis is regulated by a balance between pro- and anti- angiogenic factors. Research shows that angiogenesis and anti angiogenesis has given promising results in treatment of diseases like AIDS, atherosclerosis, ischemic heart disease and Alzheimer’s. In twenty first century, application of angiogenesis in therapeutics may have an impact on disorders similar to that which the discovery of antibiotics had in twentieth century.
REFERENCE ID: PHARMATUTOR-ART-1694
INTRODUCTION:
In simple words, the process of formation of new blood vessels is called Angiogenesis. Blood vessel formation is of two types:
• Generation of blood vessels by differentiating the Endothelial Cells (EC) is called Vasculogenesis.
• Sprouting of new blood vessels from the pre-existing ones is called Neovascularization
Angiogenesis is a natural event that occurs under both normal and pathological conditions. In the normal state, two distinct processes can be seen:
• One process utilizes endothelial progenitor cells (EPC) These are usually derived from bone marrow and initiate endothelial growth and vascular tube formation.
• The second process utilizes existing vasculature to generate new vessels, and is highly dependent on endothelial cell activation and protease secretion.
Under pathological conditions, many of the same steps involved in normal vessel formation are repeated. However, the structures formed are often functionally abnormal, possibly due to an imbalance in the angiogenic process. Multiple factors contribute to angiogenesis, including soluble growth and differentiative factors, extracellular matrix components, membrane-bound receptors, and intracellular signaling molecules. [2]
CONTROL OF ANGIOGENESIS:
[adsense:468x15:2204050025]
Angiogenesis occurs in the healthy body for healing wounds and for restoring blood flow to tissues after injury or insult. In females, angiogenesis also occurs during the monthly reproductive cycle (to rebuild the uterus lining, to mature the egg during ovulation) and during pregnancy (to build the placenta, the circulation between mother and fetus). However excessive angiogenesis may lead to disorders like cancer, atherosclerosis, diabetes, psoriasis and adiposity. These disorders can be treated by simultaneous inhibition of angiogenesis (anti angiogenic therapy) Figure1 [3]

PROCESS OF ANGIOGENESIS:
The process on angiogenesis is regulated an equilibrium between pro- and anti-angiogenic factors. The process of new blood vessels formation is divided into following steps:
* Stimulation of angiogenic response.
* Endothelial cell migration, proliferation and lumen formation.
* Tube formation.
* Maturation of basement membrane.
* Down regulation of angiogenic response.
1. Stimulation of angiogenic response:
Angiogenesis initiates in response to hypoxia which is caused by the release of hypoxia inducible factors (HIF). This results in release of angiogenic stimulators which leads to activation of Endothelial cells (EC).EC in the parent vessel alter their attachments to basement membrane & pericytes. Change in the flow, mechanical force transmitted through the endothelium activates proteases that remodel the vascular basement membrane causing it to thin & its composition.The activated endothelial cells produce matrix metalloproteinase (MMPs), a special class of degradative Enzymes. The MMPs will break down the extracellular matrix, hence permitting the migration of endothelial cells.
2. Endothelial cell (ec) migration, proliferation and lumen formation:
They both contributing increase the length of developing vessels; but migration EC forms the tip of capillary sprout. Angiogenesis factors: VEGF, FGF, modulate both proliferation & migration of EC. Complex of metaloprotease & Integrins on EC coordinate migration with exposure to matrix molecules to facilitate adhesions. As endothelial cells migrate into the surrounding tissues, they will begin to proliferate and migrate out of the existing blood vessel (Figure 2).
The lumen develops proximally in the proximally sprout as continuation of lumen from the parent vessels. Lumen formation requires the production of fibronectin & laminin matrix depends on adhesion molecules & cell surface glycoprotein. Specialized molecules called adhesion molecules, or Integrins serve as grappling hooks to help pull the sprouting new blood vessel sprout forward.
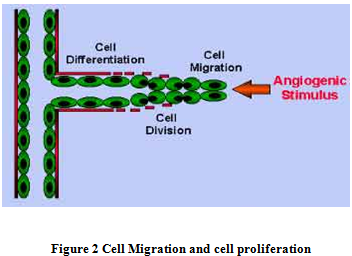
3. Tube Formation :
Parallel capillaries fuse & coalescence along their length or at their tips to form a loop capable of handling blood flow. Blood flow causes further vascular remodeling. Blood cells (like platelets) carry several endothelial factors that facilitate maturation of newly perfused vessels. At the same time, the surrounding mesenchymal cells produce a signal molecule, which binds to the receptor, Tie-2, on the surface of the endothelial cell. This stimulates the migration of the mesenchymal cells toward the vascular endothelium. These mesenchymal cells will form the vascular smooth muscle, known as pericytes, which provide structural support around the blood vessel tubes.
4. Maturation of basement membrane:
The new membrane is synthesized by the newly formed blood capillaries. Interaction between the EC, ECM and mesenchymal cells is a prime condition for the formation of a stable vasculature. The Platelet-derived Growth Factor (FDGF) regulates the recruitment of pericytes and smooth muscle cells required for the stabilization of the new blood capillaries.
5. Down regulation of angiogenic response:
Finally when sufficient neovascularization has occurred, downregulation occurs and the local concentration of angiogenic inhibitors increases. Thus, the newly formed EC becomes quiescent[4-6].
ANGIOGENIC GROWTH FACTORS:
There are many growth factors involved in the process of angiogenesis. Of these, Vascular Endothelial Growth Factor (VDGF), Fibroblast Growth Factor (FGF) and Hypoxia Inducible Growth Factor (HIGF) play an important role for cardiovascular system. They are explained briefly below:
Vascular Endothelial Growth Factor(VDGF):
Vascular endothelial growth factor (VEGF) is a potent proangiogenic factor. VEGF family consists of seven members – VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F and placental growth factor (PlGF)[7] The biological functions of VEGF are mediated upon binding to receptor tyrosine kinase, VEGFR-1, VEGFR-2, and VEGFR-3. [8] VEGF/VEGF-receptor system is a key component in the complex process of angiogenesis that also includes many other stimulators, inhibitors and angiogenic modulators.[7] VEGF is expressed in a wide variety of tissues, including brain, kidney, liver, and spleen, and by numerous cell types. VEGF influences many steps in the angiogenic response. It stimulates degradation of the extracellular matrix surrounding endothelial cells, and it promotes endothelial cell proliferation, migration, and organization into tubular structures. [9] In vivo, VEGF has been shown to regulate vascular permeability, which is important for the initiation of angiogenesis by extravasating plasma proteins that form provisional matrix favoring cell migration[3, 10].
Fibroblast Growth Factor (FGF):
FGF consist of nine types of structurally similar polypeptides characterized by their high affinity towards heparin. FGF1 (also called acidic FGF or aFGF) are present in brain and retina. They are not present on cardiac tissue. FGF2 (also called basic FGF or bFGF) are widely distributed in tissues including cardiac tissue. Various studies on animal models have shown that aFGF has no effect on myocardial angiogenesis but bFGF when administered through intracoronary route shows effective neovascularization. In vitro, FGF is a potent stimulator of endothelial cell proliferation. It is more promiscuous than VEGF in that FGF also stimulates fibroblast and smooth muscle cell proliferation thus leading to the formation of capillaries as well as arterioles. In contrast to VEGF, FGF has no effect on vascular permeability [11-13].
Hypoxia Inducible Factor (HIF):
Tissue oxygenation represents a steady state based upon O2 consumption, primarily by mitochondrial oxidative phosphorylation, and O2 delivery via erythrocytes traveling through tissue capillary networks. This steady state represents a balance between the requirement for O2 as an energy substrate and the inherent risk of oxidativedamage to cellular macromolecules [14] .Hypoxia-inducible factor-1 (HIF-1) is a heterodimeric α, β-transcriptional complex that mediates the cellular response to oxygen availability in multicellular organisms. Dimerisation of the HIF-α and HIF-β subunits under hypoxic conditions activates the transcription of an array of genes involved in the adaptation of cells to the lowered oxygen tension. Medicinal stimulation of the natural HIF mediated response might be used to treat ischemic illnesses such as heart disease; in contrast, inhibition of the HIF mediated hypoxic response might be used for the treatment of tumors via inhibition of angiogenesis. Short-term hypoxia can also elevate platelet numbers, while prolonged exposure may cause some degree of thrombocytopenia in response to increased levels of erythropoietin (EPO) [15].
ANGIOGENIC THERAPY IN MYOCARDIAL ISCHEMIA:
Tissues depend on oxygen and nutrients for their metabolic needs. Because there is a threshold limit for the diffusion of these elements, vessels need to grow whenever cells increase in number or size. Accordingly, muscular hypertrophy is associated to physiologic neovascularization; while vessels tend to regress under conditions of non-use [16]. The healing of damaged tissues represents one prototypical situation in which the rapid formation of new vessels is urgently required.The other way round, in patients with limb ischemia, supply side of angiogenic factors is thought to benefit tissue repair by compensating a putative endogenous deficit in production/ release.
Currently available approaches for treating coronary arterial disease aim to relieve symptoms either by reducing myocardial oxygen demand, preventing further disease progression, restoring blood flow a large localized segment of the epicardial coronary arterial tree. Recent advances in our understanding of the stimuli and control mechanism governing the development of new blood vessels in coronary heart disease have led to an improved picture of compensatory healing process the accompanies myocardial ischemia and infraction. Two areas of particular recent interest include:
- Mechanism of blood vessel formation in ischemic coronary disease
- Therapeutic application of angiogenic growth factors in animal models and in patients[17]
Angiogenesis triggering factors:
It is a very complex process. Conceptually the major triggers of this process can be simplified into three broad categories: Mechanical, Chemical and Molecular factors (Figure 3).
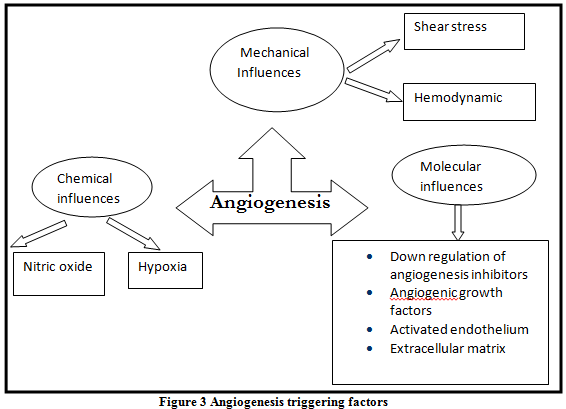
* Mechanical influences:
Hemodyanmics: Augmentation of blood flow during exercise, the hyperthyroid state (Thyrotoxicosis) and administration of certain drugs have all been shown to stimulate vascular sprouting [20]
Shear stress: Shear stress has an important influence on the development of collaterals in the ischemic tissues. Shear stress and stretch of the myocardium (i.e. increase left ventricular end diastolic volume) can lead to up-regulation of adhesion molecules in the endothelium, attraction of the inflammatory cells and stimulation of the endothelial cells to produce angiogenic factors [21, 22]
*Chemical influences:
This can happen either through hypoxia or through the production of nitric oxide:
Hypoxia and oxygen tension gradient: Hypoxia stimulates macrophages to release various factors including platelets derived growth factor and fibroblast growth factor 1 and 2 Hypoxia has been shown to upregulate vascular endothelial growth factor owing both by increasing transcription mediated by hypoxia inducible factor 1 and by increasing the stability of vascular endothelial growth factor mRNA Hypoxia also increase the expression of vascular endothelial growth factor receptors (FLK and FLT). [23]
Nitric oxide: VEGF is known to induce the release of Nitric oxide from endothelial cells, and vascular endothelium inducible NO synthase (iNOS), production is amplified during VEGF- induced angiogenesis. Nitric Oxide has also a regulatory effect on VEGF production [24, 25].
* Molecular influences:
Role of growth factor receptors: Administration of growth factors in the normal heart does not result in angiogenesis. This phenomenon has been explained by the lack of expression of appropriate growth factor receptors within the normal tissue. Studies of tumor angiogenesis have documented that up-regulation of receptor density is an important modulatory mechanism in angiogenesis. It has been shown that the FLK and FLT (vascular endothelial growth factor) receptors are up-regulated by hypoxia and, possibly, by the presence of other growth factors. Vascular endothelial growth factor and fibroblast growth factor, when administered simultaneously, potentiate one another’s effect perhaps reflecting reciprocal up-regulation of their respective receptors [24].
Down-regulation of angiogenic inhibitors:
The presence of angiogenic growth factors and their respective receptors have been demonstrated in normal tissue. Despite the coexistence of both angiogenic ligands and their receptors in normal tissues, persistent, active neovascularization is not typically observed. This implies the influence of an angiogenic inhibitor. Induction of the cascade of events that leads to angiogenesis would, therefore, require the appropriate downregulation of the inhibitory pathway.
THERAPEUTIC ANGIOGENESIS:
The high-affinity endothelial receptors for various angiogenic growth factors are now well-recognized to mediate proliferation, migration and differentiation of the cellular populations required for the augmented growth of new vascular structures in ischemic tissues. Therapeutic angiogenesis is the clinical use of methods to enhance or promote the development of collateral blood vessels in ischemic tissue. Angiogenic treatment is potentially important as we see more patients that are having ischemia or vascular insufficiency that are not amenable to revascularization. This new form of treatment is an alternative to high risk percutaneous coronary intervention or coronary artery bypass surgery. [19]
Strategies for drug delivery in therapeutic angiogenesis [23]
Therapeutic angiogenesis can be affected by one of two mechanisms of drug delivery (Table 1):
- Transfer of the relevant genetic material
- Therapeutic administration of the angiogenic factor itself
|
Gene transfer |
Native factor |
|
|
Table 1: various modes of drug delivery
Rationale for therapeutic angiogenesis in patients with occlusive vascular diseases:
There are two possible explanations for occlusive vascular disease: either the production of angiogenic cytokines is inadequate, or patients’ response to them is attenuated. It has been shown that angiogenesis is impaired in older v younger animals secondary to reduced VEGF expression (Table 2).
Reduced expression of angiogenic cytokines is not the only factor contributing to the heterogeneous response to collateral vessel development. Marked genetic heterogenecity in the response to growth factor-stimulated angiogenesis has been observed in different strains of inbred mice[18]. Age-related reduction in endothelial cell viability has also recently been demonstrated. Endothelial dysfunction accompanies many of the known coronary risk factors, and may reduce endothelial responsiveness to angiogenic growth factors.
|
Growth factors |
Molecular Targets |
Effects on Progenitor Cells |
|
VEGF
|
VEGF receptors expressed on endothelial cells, monocytes, hematopoietic stem cells; stimulates proliferation, migration, and tube formation |
Mobilization of EPC Improves survival and differentiation of EPC |
|
FGF
|
FGF receptors expressed on endothelial cells smooth muscle cells, and myoblasts; stimulates proliferation. |
Included in EPC culturing media |
|
Angiopoietin-1
|
Tie-2 receptor expressed on endothelial cells; enhances vessel maturation and stability |
Mobilizes EPC and hematopoietic progenitor cells
|
|
Chemokines MCP-1 |
Promotes arteriogenesis by stimulating CCR-2 receptor on monocytic cells. |
Chemoattractant for EPC.
|
Table 2 Factors Augmenting Neovascularization: Potential Candidates for Therapeutic Angiogenesis, Arteriogenesis, and Vasculogenesis
Strategies to increase EC responsiveness and to enhance EPC production or availability are therefore reasonable targets for therapeutic angiogenesis [26, 27]
Gradual occlusion of coronary arteries is frequently associated with development of collateral circulation and the net effect is rarely adequate to compensate fully for the flow lost to occlusion of native epicardial coronary arteries. Candidates for pharmacological stimulation of therapeutic angiogenesis in cardiac ischemia include angiogenic cytokines such as fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), Hepatocyte growth factor, growth factors involved in maturation of the vascular tree such as Angiopoietin and platelet derived growth factor, CXC chemokines such as interleukin 8 and monocyte chemoattractant protein-1, and transcriptional factors that stimulate expression of angiogenic cytokines and their receptors such as hypoxia inducible factor (HIF) . [28]
Therapeutic angiogenesis is not free from potential harmful effects. Animal studies and clinical trials suggested hypotension is associated with both bFGF and especially VEGF administration due to nitric oxide release and arteriolar vasodilatation. [27] Concerns associated with angiogenic growth factors include plaque angiogenesis, proliferative retinopathy and occult malignancies. [29] Since potent angiogenic growth factors may have grave side effects, a high drug target level and low systemic exposure should be the ultimate goal. It is suggested that intramyocardial delivery of growth factors would be preferred since this would include the potential to target specific areas of the heart, likely higher efficacy of delivery, and prolonged tissue retention [30]. There are three major ways to promote angiogenesis:[19]
• Protein Therapy.
• Gene Therapy.
• Cellular Therapy.
* Protein Therapy
Angiogenesis can be achieved by growth factor proteins. These factors include VEGF, bFGF through different routes. The argument in favor of protein therapy are Finite temporal exposure to angiogenic factor, titratable dosing of angiogenic factor exposure, no exposure to exogenous genetic material, no exposure to viral vector, readministration may be easier; decreased risks of inflammatory response or immune inactivation at the time of repeat dosing, modulation of protein structure and/or combination with slow- release delivery system may abrogate issue of protein stability. Recent advances in drug delivery methods using bioerodible polymer matrices will allow long-term sustained release of the growth factors. This will resolve one of the major problems associated with protein administration; namely, the short serum half life and so tissue half life and that would lead to the need of frequent administration and higher doses. An important consideration, however, is that protein therapy is limited to secreted factors. Delivery of intracellular modulators for therapeutic angiogenesis, including transcription factors that control angiogenesis such as hypoxia inducible factor-1 alpha (HIF-1α), is only possible through gene therapy [31].
* Gene Therapy:
Gene therapy is known as any manipulation of gene expression or function for the treatment of specific disease. The advantages of gene therapy are, sustained production of angiogenic factor resulting in prolonged exposure to elevated level, the ability for local delivery and so less systemic exposure. This all can be done with one dose as well and the production of angiogenic factor can be directed to specific cell type and so less side effects.
The disadvantages for gene therapy include:
- Introduction of foreign genetic material.
- Exposure to viral vectors with concomitant risk of inflammatory response, viral persistence, and in vivo recombination.
- Potential for non- targeted cell gene delivery.
- Inability to precisely modulate gene expression and thus angiogenic factor levels with present vector system.
- Potential for inactivation and/or inflammatory response at readministration.[19]
* Cellular Therapy:
This is a new approach in which cells that are known to produce angiogenic factors are used to promote angiogenesis in ischemic tissue [32]. Many cell types have been used for this purpose as monocyte and endothelial progenitor cells and marrow stromal cells. These cells have been shown to stimulate angiogenesis through expression of growth factors stimulated by local paracrine stimuli from ischemia. These cells can also participate as cellular precursors for Vasculogenesis and they can act as a vehicle for delivery of therapeutic genes coding for angiogenic factors[19].
POTENTIAL RISKS OF INDUCING ANGIOGENESIS:
The risks associated with therapeutic angiogenesis include those that are specific to the growth factor per se, and those generic to strategies of promoting angiogenesis. With regard to the former, risks thus far recognized clinically in association with VEGF depend upon the mode of administration. Administration of recombinant protein may lead to hypotension due to the fact that VEGF up regulates nitric oxide synthesis. A third issue concerns the development of angiomata in mice or rats treated with transduced myoblasts or supraphysiologic doses of plasmid DNA respectively. Importantly, in the latter study, no angiomata were observed when the dose of administered plasmid DNA was reduced by 50%[18]
CONCLUSION:
Research in angiogenesis is showing a marked impact on the therapeutic field. The hope of generating new functional blood vessels in ischemic disease is becoming more realistic every day. Better understanding and linear research will enable the scientists to develop new drugs and therapies that are able to cure life threatening diseases like cancer, AIDS and cardiovascular diseases.
REFERENCES:
1. The angiogenesis foundation, Understanding angiogenesis (document on internet). angio.org.in/ua.php
2. rndsystem.com – Angiogenesis and Vasculogenesis.
3. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat med1995; 1:27-31.
4. Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol 2002;282:C947-70
5. Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in performed and newly performed blood vessels during tumor angiogenesis. Microvasc Res 1977;14:53-65
6. Bischoff J. Cell adhesion and angiogenesis. J Clin Invest 1997;100:S37-9
7. Himadri Roy, Shalini Bhardwaj, Seppo Yla¨-Herttuala “Biology of vascular endothelial growth factors” FEBS Letters;2006; 580; 2879–87
8. Jade Homsi, , Adil I. Daud. Spectrum of Activity and Mechanism of Action of VEGF/PDGF Inhibitors. Journal of the Moffitt Cancer Center. 2007; 14(3):285-295.
9. Peter J. Polverini. Angiogenesis in Health and Disease Insights into Basic Mechanisms and Therapeutic Opportunities. Journal of Dental Education 2002; 66(8):962-976.
10. Sandra Liekens, Erik De Clercq, Johan Neyts. Angiogenesis: regulators and clinical applications. Biochemical Pharmacology 2001;61;253–270
11. Unger EF, Shou M, Sheffield CD et al. Extracardiac to coronary anastomoses supports regional left ventricular function in dogs. Am J Physiol 1993; 264 (5 Pt 2): H 1567-74.
12. Unger EF, Banai S, Shou M et al. A model to assess interventions to improve collateral blood flow: Continuous administration of agents into the left coronary artery in dogs. Cardiovasc Res 1993; 27 (5): 785-91.
13. Battler A, Scheinowitz M, Bor a et al. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infracted swine myocardium. J Am Coll Cardiol 1993; 22 (7): 2001-06.
14. Gregg L. Semenza. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. TRENDS in Molecular Medicine 2001; 7 (8):345-351.
15. Schofield and Ratcliffe. Oxygen sensing by HIF hydroxylases. Nature Reviews Molecular Cell Biology 2004; 5: 343-354.
16. Sandri, M., et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes results of three randomized studies. Circulation 2005;111;3391–3399
17. Napoleone ferrara, Robert S. kerbel. Angiogenesis as a therapeutic target. Nature 2005; 438; 967-974.
18. Saul Benedict Freedman, Jeffrey M. Isner. Therapeutic Angiogenesis for Ischemic Cardiovascular Disease. J Mol Cell Cardiol 2001; 33,;379–393
19. Hilal Al Sabti. Therapeutic angiogenesis in cardiovascular disease. Journal of Cardiothoracic Surgery 2007;2;49-56
20. Gibaldi M: Regulating angiogenesis: A new therapeutic strategy. J Clin Pharma 1998; 38; 898-903.
21. Nagel T, Resnick N, Atkinson WJ, Dewey CF Jr, Gimbrone MA Jr: Shear stress selectively upregulate intracellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest 1994; 94; 885-891.
22. Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D: Vascular permeability factor: A tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity and promotes monocyte migration. J Exp Med 1990; 172;1535-45
23. R. Tabibiazar and S. G. Rockson. Angiogenesis and the ischemic heart. European Heart Journal 2001; 22;903–918
24. Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R: Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997; 99; 2625-34.
25. Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T: Endogenous nitric oxide synthase inhibitor: A novel marker of atherosclerosis. Circulation 1999;99;1141-6
26. Isner JM. Tissue responses to ischemia: local and remote responses for preserving perfusion of ischemic muscle. J Clin Invest. 2000; 106: 615–619.
27. Isner JM, Asahara T. Angiogenesis and Vasculogenesis as therapeutic strategies for postnatal vascularization J Clin Invest. 1999; 103: 1231–1236.
28. Michio Kawasuji. Therapeutic Angiogenesis for Ischemic Heart Disease. Ann Thorac Cardiovasc Surgi 2002; 8 (2); 59-61.
29. Simons M, Bonnow RO, Chronos NA, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation 2000; 102: e73–86.
30. Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc Res 2001; 49: 522–31.
31. Yin-Shan Ng and Patricia A D’Amore. Therapeutic angiogenesis for cardiovascular disease. Curr Control Trials Cardiovasc Med 2001; 2; 278-285.
32. Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH: Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation 2001; 104; I207-12.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 1, Issue 1 Received On: 18/10/2013; Accepted On: 03/11/2013; Published On: 25/11/2013 How to cite this article: JH Thakkar, AA Mansuri, C Patel, GK Jani, Angiogenesis in Myocardial Ischemia: An Organized Review, PharmaTutor, 2013, 1(1), 65-74 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









