About Author
Ekta Khare*, Saswati Sharma, Yuvraj Singh, Priyanka Keshri
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, I.T.M. University, Gwalior,
Madhya Pradesh-474001, India
ektakhare23@gmail.com
ABSTRACT :
As of late heterocyclic mixes analogs and subordinates have pulled in wide consideration because of their helpful natural and pharmacological properties. Benzothiazole is among the typically happening heterocyclic cores in numerous marine just as characteristic plant items. Benzothiazole is a special bicyclic ring framework with various applications. It is known to show a wide scope of organic properties including anticancer, antimicrobial, and antidiabetic, anticonvulsant, calming, antiviral, antitubercular exercises. An enormous number of restorative specialists are combined with the assistance of benzothiazole core. During late years there have been some intriguing advancements with regards to the natural exercises of benzothiazole subsidiaries. These mixes have unique centrality in the field of therapeutic science because of their exceptional pharmacological possibilities. This audit is mostly an endeavor to introduce the examination work revealed in the ongoing logical writing on various organic exercises of benzothiazole mixes.
INTRODUCTION:
Benzothiazole is an organic heterocyclic ring system that is a chemical result of the fusion between benzene and thiazole. Its molecular weight is 135.19 g/mol. Its derivatives were found to be possessing and exhibiting a wide range of astounding medicinal properties some of them being anticancer, antimicrobial, antidiabetic, anticonvulsant, anti-inflammatory, antiviral, antitubercular, antihelminthic, and anti-fungal, anti-malarial. As BT demonstrates such a wide spectrum of activity it for sure is very important for drug development. Its derivatives are believed to be useful for the treatment of diseases like brain ischemia, several neurogenerative disorders, Huntington's disease, and Cancer.
METHOD:
A literature search was conducted on various database sources (like PubMed, ScienceDirect) with the help of a combination of different keywords such as Benzothiazole, thiazole, antitumor, anti-inflammatory activity, anticonvulsant, antioxidant, anti-mutagenic, anti-diabetic, anti-hyperplasia, and antimicrobial. The search was customized by applying the appropriate filter to get the most relevant articles to meet the objective of this review.
Clinical Activity:
Anticancer activities:
After cardiovascular diseases, Cancer is the 2nd largest disease worldwide which causes the majority of deaths. The BT nucleus with various substitutions shows antitumor activity. Substituted 2-(4-aminophenyl)benzothiazole is one such compound that represents a potent, highly selective, and new class of antitumor agents. After a thorough examination, it shows antitumor activity in human cell lines of breast, colon, lung, ovarian, and renal carcinoma.
According to the literature study of Benzothiazole derivatives as anticancer agents are
• Fluorinated derivatives
• Imidazole based benzothiazole derivatives
• Piperazine based benzothiazole derivatives
• Oxadiazole based benzothiazole derivatives
• Morpholine- thiourea based benzothiazole derivatives
• Thiophene based benzothiazole derivatives
• Thiadiazole based benzothiazole derivatives
• Substituted pyridine based benzothiazole derivatives
• Pyrazole based benzothiazole derivatives
• Pyrimidine based benzothiazole derivatives
• Piperidine based benzothiazole derivatives
• Secondary sulphonamide benzothiazole derivatives
• Benzamide based benzothiazole derivatives
• Quinolone based benzothiazole derivatives
Benzothiazole derivatives that show carbonic anhydrase inhibitory and antitumor action are sulphonamides, their isosteres, and their inorganic ions.
Antimicrobial activity:
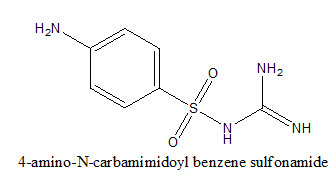
U.K. Singh et.al reported the synthesis of Schiff's and N-Mannich bases of isatin and its derivatives with 4-amino-N-carbamimidoyl benzene sulfonamide3 was tested for antibacterial activity by MIC method on strains S.aureus, B.pumulis, B.subtilis, E.coli, S.abony, K pneumonia.These compounds exhibited very significant and better antibacterial activity.
Ramachandran et al. reported the synthesis of Schiff and mannich bases of Isatin derivatives. These were also tested for anti-microbial activity but by cup and plate method on strains of staphylococcus aureus, streptococcus pyogenes, Escherichia coli, klebsilla aerogenes, and candida albicans. Most of them displayed greater antibacterial and antifungal activities when compared to standard drugs.
Antitubercular activity:
Tuberculosis is a serious bacterial infection that affects the lungs and spreads easily meaning it is highly contagious. Even though it is partly preventable, the keyword here is partly. Scientists all over the world are researching this topic.
It was found that H37Rv and MDR-MTB were the 2 strains of Mycobacterium tuberculosis against which the benzothiazole analogs were tested. MDR is multidrug-resistant M.tuberculosis which is resistant to isoniazid, rifampicin, and ethambutol. The testing was done by the Agar method.
![{2-(benzo[d]thiazol-2-yl-methoxy)-5-fluorophenyl}-(4-chlorophenyl)-methanone](https://1.bp.blogspot.com/-LRllndWgNZU/YEbpD2nfQcI/AAAAAAAALHE/OjjBDBXjxwIZ_qJPwgvEMd4yrAuy1yK-QCLcBGAsYHQ/s553/H37Rv-and-MDR-MTB.jpg)
Bhusari KP et al. prepared and reported that {2-(benzo[d]thiazol-2-yl-methoxy)-5-fluorophenyl}-(4-chlorophenyl)-methanone was the compound that displayed impressive activity against H37Rv and MDR-MTB with minimum inhibitory concentrations.
Antiviral activity:
Due to vigorous in-silico virtual and HTS which resulted in higher hit-to-lead momentum the anti-viral potential of benzothiazole derivatives were discovered. New series of Iamvudine prodrugs involving N4-substitution with Isatin derivatives showed in-vitro antiretroviral activities.
Anti-malarial activity:
According to Structure-activity relationship studies, nitrogen and sulfur substituted five-membered aromatic ring present within benzothiazole hydrazones might be responsible for their antimalarial activity.
Hout S et al. also reported that tests on the strains W2 and 3D7 of P. falciparum for Antimalarial activity of 2-substituted-6-nitro, 6-amino benzothiazoles, and their anthranilic acids were positive and the results revealed the potency of compounds as the antimalarial agents of clinical and biological research.
Anti- Inflammatory activity:
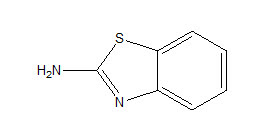
According to the literature survey, 2-amino-benzothiazole is a versatile compound and could be a potential pharmacophore in safe anti-inflammatory agents. It was noted that when the 2- amino benzothiazole is substituted at 4 or 5 positions with electron-withdrawing groups like Cl, NO2, OCH3, an increase in anti inflammatory activity was found.
Antileishmanial activity:
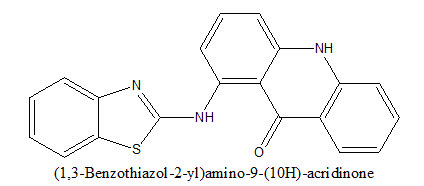
Delmas et al.8 synthesized (1,3-Benzothiazole-2-yl)amino-9-(10H)-acridinone derivatives via a procedure based on the Ullman reaction.
Anti- Convulsant activity :
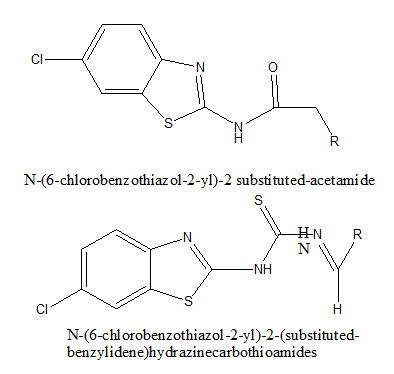
Amir et al. reported a new series of N-(6-chlorobenzothiazol-2-yl)-2-substituted-acetamide and N-(6-chlorobenzothiazol-2-yl)-2-(substituted-benzylidene)hydrazine-carbothioamides.
Kumar et al. have designed and synthesized a series of 2-[2-(substituted)hydrazinyl]-1,3-benzothiazole and 2-(1,3-benzothiazol-2-yl sulfanyl)-N-(substituted) acetohydrazide.The anticonvulsant activity of the titled compounds was assessed using the 6 Hz psychomotor seizure test.
Conclusion:
The current survey features the utilization of benzothiazole moiety as a format for the improvement of fresher restorative specialists. Organic properties of the core incorporate anticancer, antidiabetic, pain-relieving, calming, and antimicrobial. With legitimate planning and structure-movement relationship investigations of known benzothiazole, forthcoming mixes can be planned and combined for an assortment of organic exercises.
REFERENCES:
1. Z. Wang, X. Shi, J. Wang, et al., "Synthesis, structure-activity relationships and preliminary antitumor evaluation of benzothiazole-2-thiol derivatives as novel apoptosis inducers," Bioorganic and Medicinal Chemistry Letters, vol. 21, no. 4, pp. 1097–1101, 2011.
2. L. Jin, B. Song, G. Zhang, et al., "Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)-O, O-dialkyl-α-amino phosphonates,"Bioorganic and Medicinal Chemistry Letters, vol. 16, no. 6, pp. 1537–1543, 2006.
3. R. M. Kumbhare, T. Dadmal, U. Kosurkar, V. Sridhar, and J. V. Rao, “Synthesis and cytotoxic evaluation of thiourea and N-bis-benzothiazole derivatives: a novel class of cytotoxic agents,” Bioorganic and Medicinal Chemistry Letters, vol. 22, no. 1, pp. 453–455, 2012.
4. Kamal, K. S. Reddy, M. N. Khan et al., “Synthesis, DNA-binding ability and anticancer activity of benzothiazole/benzoxazole-pyrrolo[2,1-c][1,4]benzodiazepine conjugates,” Bioorganic and Medicinal Chemistry, vol. 18, no. 13, pp. 4747–4761, 2010.
5. D. T. Oanh, H. V. Hai, S. Park et al., “Benzothiazole-containing hydroxamic acids as histone deacetylase inhibitors and antitumor agents,” Bioorganic and Medicinal Chemistry Letters, vol. 21, no. 24, pp. 7509–7512, 201
6. F. Delmas, A. Avellaneda, C. D. Giorgio et al., “Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives,” European Journal of Medicinal Chemistry, vol. 39, no. 8, pp. 685–690, 2004
7. Dong- Fang S., Bradshaw, T.D., Wrigley, S., McCall, C.J., Lelieveld, P., Fitchner, I. and Stevens, M.F.G., J. Med. Chem. , 1996, 39, 3375.
8. Kochichiro, Y., Katsumi, G., Kazuya, Y., Tominori, M. and Goro, T., J. Med. Chem. , 1990, 33, 2192.
9. Bhusari, K.P., Khadekar, P.B., Umathe, S.N., Bahekar, R.H. and Rao, A.R., Indian. J. Heterocycl. Chem. , 2000, 9, 213.
10. Klein, L.L., Yeuns, C.M., Weissing, D.E., Lartey, P.A., Tonaka, S.K., Plattner, J.J. and Mulford, D.J., J. Med. Chem., 1994, 37, 572.
11. Verma, R.S. and Singh, A.P., Indian. J. Chem. , 1998, 27B, 438.
12. Mei-Sze C., Dong- Fang S., Wrigley, S., Bradshaw, T.D., and Hutchinson, I., Shaw, P.N., Barrett, D.A., Stanley, L.A. and Stevens, M.F.G., J. Med. Chem., 1999, 42, 381.
13. Goldin, A., Schepartz, S.A., Venditti, J.M. and Devita, V.T., In; Methods in Cancer Research, Vol.16, Academic Press, 1979, 165.
14. Rajeeva B, Srinivasulu N and Shantakumar S: Synthesis and antimicrobial activity of some new 2-substituted benzothiazole derivatives. E-Journal of Chemistry 2009; 6 (3): 775-779.
15. Kini S, Swain S and Gandhi A: Synthesis and evaluation of novel benzothiazole derivates against human cervical cancer cell lines. Ind J Pharm Sci 2007; 69(1): 46-50.
16. Stanton HLK, Gambari R, Chung HC, Johny COT, Filly C and Albert SCC: Synthesis and anti-cancer activity of benzothiazole containing phthalimide on human carcinoma cell lines. Bioorg Med Chem 2008; 16: 3626-3631.
17. Wang M, Gao M, Mock B, Miller K, Sledge G, Hutchins G, and Zheng Q: Synthesis of C-11 labeled fluorinated 2-aryl benzothiazoles as novel potential PET cancer imaging agent. Bioorg Med Chem 2006; 14: 8599-8607.
18. Sathe BS, Jayachandran E, Jagtap VA, and Sreenivasa GM: Anthelmintic activity of newly synthesized moieties of fluoro benzothiazole Schiff’s bases. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2011; 2 (1): 510-515.
19. Pattan S, Suresh C, Pujar V, Reddy V, Rasal V and Koti B: Synthesis and antidiabetic activity of 2-amino[5”(4-sulphonylbenzylidine)-2, 4-thiazolidinenone]-7-chloro-6-flurobenzothiazole. Ind J Chem 2005; (44B): 2404-2408.
20. Reddy P, Lin Y and Chang H: Synthesis of novel benzothiazole compounds with an extended conjugated system. Arcivoc 2007; 16: 113-122.
21. Heo Y, Song Y, Kim B and Heo J: A highly regioselective synthesis of 2-aryl-6-chlorobenzothiazoles employing microwave-promoted Suzuki-Miyaura coupling reaction. Tetrahedron Letters 2006; 47: 3091-3094.
22. Shinde PK and Waghamode KT: Synthesis, characterization and antibacterial activity of substituted benzothiazole derivatives. International Journal of Scientific and Research Publications 2017; 7(8): 365-370.
23. Maddila S, Gorle S, Seshadri N, Lavanya P and Jonnalagadda SB: Synthesis, antibacterial and antifungal activity of novel benzothiazole pyrimidine derivatives. Arabian Journal of Chemistry 2016; 9(5): 681-687.
24. Bele DS, Kothari H and Singhvi I: Synthesis and antimicrobial activity of some benzothiazole derivatives. International Journal of Pharmaceutical and Chemical Sciences 2012; 1(4): 1238-1242.
25. Padalkar VS, Borse BN, Gupta VD, Phatangare KR, Patil VS, Umape PG and Sekar N: Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arabian Journal of Chemistry 2016; 9(2): S1125-S1130.
26. Soni B, Ranawat MS, Sharma R, Bhandari A and Sharma S: Synthesis and evaluation of some new benzothiazole derivatives as potential antimicrobial agents. Eur J Med Chem 2010; 45: 2938-2942.
27. Geronikaki A, Argyropoulou I, Vicini P and Zani F: Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents ARKIVOC 2009; 6: 89-102.
28. Jagtap VA, Jaychandran E, Sreenivasa GM and Sathe BS: Synthesis of some fluoro substituted sulphonamide benzothiazole comprising thiazole for anti-microbial screening. International Journal of Pharma and Bio Sciences 2010; 1(4): 484-490.
29. Amir M, Kumar A, Ali I and Khan S: Synthesis of pharmaceutical important 1, 3, 4-thiadiazole and imidazolinone derivatives as antimicrobials. Ind J Chem 2009; 48B: 1288-1293.
30. Murthi Y and Pathak D: Synthesis and Antimicrobial screening of Substituted 2-Mercaptobenzothiazoles. J Pharm Res 2008; 7(3): 153-155.
31. Osmaniye D, Levent S, Karaduman AB, Ilgın S, Ozkay Y and Kaplancıkl ZA: Synthesis of new benzothiazole acylhydrazones as anticancer agents. Molecules 2018; 23: 1054-1068.
32. Saipriya D, Prakash A, Kini SG, Bhatt GV, Pai KSR, Biswas S and Shameer KM: Design, synthesis, the antioxidant and anticancer activity of novel Schiff's bases of 2-amino benzothiazole. Indian Journal of Pharmaceutical Education and Research 2018; 52(4s): S333-S342.
33. Uremis N, Uremis MM, Tolun FI, Ceylan M, Doganer A and Kurt AH: Synthesis of 2-substituted benzothiazole derivatives and their in-vitro anticancer effects and antioxidant activities against pancreatic cancer cells. Anticancer Research 2017; 37: 6381-6389.
34.
35. Lindgren EB, De Brito MA, Thatyana RA, Vasconcelos, de Moraes MO, Montenegro RC, Yoneda JD and Leal KAZ: Synthesis and anticancer activity of (E)-2-benzothiazole hydrazones. European Journal of Medicinal Chemistry 2016; 86: 12-16.
36. Saeed S, Rashid N, Jones PG, Ali M and Hussain R: Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur J Med Chem 2010; 45: 1323-1331.
37. Prabhu PP, Panneerselvam T, Shastry CS, Sivakumar A and Pande SS: Synthesis and anticancer evaluation of 2-phenyl thiaolidinone substituted 2-phenyl benzothiazole-6-carboxylic acid derivatives. Journal of Saudi Chemical Society 2012; 19(2): 181-185.
38. Song B, Jin L, Zhang G, Xu R, Zhang S, Gao X, Hu D and Yang S: Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluoro phenyl)-O, O-dialkyl-alpha-aminophosphonates. Bioorg Med Chem Letters 2006; 16: 1537-1543.
39. Devmurari VP, Pandey S, Goyani MB, Nandanwar RR, Jivani NP and Perumal P: Synthesis and anticancer activity of some novel 2-substituted benzothiazole derivatives. International Journal of Chem Tech Research 2010; 2: 681-689.
40. Sadhasivam G and Kulanthai K: Synthesis, charac-terization, and evaluation of anti-inflammatory and anti-diabetic activity of new benzothiazole derivatives. Journal of Chemical and Pharmaceutical Research 2015; 7(8): 425-431.
41. Verma AK, Martin A and Singh AK: Synthesis, Characterization and evaluation of Anti-inflammatory and Analgesic activity of Benzothiazole derivatives. Indian Journal of Pharmaceutical and Biological Research (IJPBR) 2014; 2(3): 84-89.
42. Mir F, Shafi S, Zaman MS, Kalia NP, Rajput VS, Mulakayala C, Mulakayala N, Khan IA and Alam MS: Sulfur rich 2-mercaptobenzothiazole and 1,2,3-triazole conjugates as novel antitubercular agents. European Journal of Medicinal Chemistry 2014; 76: 274-243.
43. Venkatesh, P and Pandeya SN: Synthesis, characterization and anti-inflammatory activity of some 2-amino benzothiazole derivatives. International Journal Chem. Tech. Res 2009; 1(4): 1355.
44. Gurupadyya B, Gopal M, Padmashali B and Manohara Y: Synthesis and pharmacological evaluation of azatidin-2-ones and thiazolidine-4-ones encompassing benzothiazole. Indian J Pharm Sci 2008; 70(5): 572-577.
45. Siddiqui N, Alam SM, Sahu M, Naim MJ, ShaharYar M and Alam O: Design, synthesis, anticonvulsant evaluation and docking study of 2-[(6-substituted benzo[d]thiazol-2-ylcarbamoyl) methyl]-1 -(4-substituted phenyl) isothio-ureas. Bioorganic Chemistry 2017; 1-31.
46. Raju GN and Nadendla RR: Synthesis and anticonvulsant activity of newer benzothiazole derivatives. World Journal of Pharmacy and Pharmaceutical Sciences 2017; 6(4): 1701-1713.
47. Liu D, Zhang H, Jin C and Quan Z: Synthesis and biological evaluation of novel benzothiazole derivatives as potential anticonvulsant agents. Mole 2016; 21(3): 164.
48. Rana D, Siddiqui N, Khan SA, Haque SE and Bhat MA: N-{[(6-substituted-1, 3-benzothiazole-2-yl) amino] carbonothioyl}-2/4-substituted benzamides: synthesis and pharmacological evaluation. Eur J Med Chem 2008; 43: 1114-1122.
49. Amnerkar ND, Bhongade BA and Bhusari KP: Synthesis and biological evaluation of some 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1, 3-thiazole-2-amines and their Schiff bases. Arabian Journal of Chemistry 2015; 8(4): 545-552.
50. Jimonet P, Francois A, Barreau M, Blanchard JC and Boirean A: Riluzole Series. Synthesis and in-vivo “antiglutamate” activity of 6-Substituted-2-benzothiazole amines and 3-Substituted-2-imino-benzothiazolines Indian J Med Chem 1999; 42(15): 2828-2843.
51. Amin S and Parle A: Synthesis, characterization and antioxidant activity of 2-aryl benzothiazole derivatives. International Journal of Current Pharmaceutical Research 2018; 10(5): 3-8.
52. Racane L, Cindric M, Perin N, Roskaric P, Starcevic K, Masek T, Mauric M, Dogan J and Karminski-Zamola G: Synthesis and antioxidative potency of novel amidino substituted benzimidazole and benzothiazole derivatives. Crotica Chemica Acta 2017; 90(2): 187-195.
53. Cabrera-Perez LC, Padilla-Martınez II, Cruz A, Mendieta -Wejebe JE, Tamay-Cach F and Rosales-Hernandez MC: Evaluation of a new benzothiazole derivativewith antioxidant activity in the initial phase of acetaminophen toxicity. Arabian Journal of Chemistry 2016; 1-12.
54. Guzel O, Karali N, Ozsoy N, Ozbey S and Slman A: Synthesis of new spiroindolinones incorporating a benzothiazole moiety as antioxidant agents. European Journal of Med Chem 2010; 45: 1068-1077.
55. Cressier D, Prouillac C, Hernandez P, Amourette C, Diserbo M, Lion C and Rima G: Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorganic & Medicinal Chemistry 2009; 17: 5275-5284.
56. Kumar S, Rathore DS, Garg G, Khatri - K, Saxena R and Sahu SK: Synthesis and evaluation of some 2-((benzo thiazol-2-ylthio) methyl)-5-phenyl-1, 3, 4-oxadiazole derivatives as antidiabetic agents. Asian Pacific Journal of Health Sciences 2016; 3(4): 65-74.
57. Meltzer-Mats E, Babai-Shani G, Pasternak L, Uritsky N, Getter T, Viskind O, Eckel J, Cerasi E, Senderowitz H, Sasson S and Gruzman A: Synthesis and mechanism of hypoglycemic activity of benzothiazole derivatives. Journal of Medicinal Chemistry 2013; 56(13): 5335-5350.
58. Mariappan G, Prabhat P, Sutharson L, Banerjee J, Patangia U and Nath S: Synthesis and antidiabetic evaluation of benzothiazole derivatives. Journal of the Korean Chemical Society 2012; 56 (2): 251-256.
59. Khokra SL, Arora K, Mehta H, Aggarwal A and Yadav M: Common methods to synthesize benzothiazole derivatives and their medicinal significance: a review. International Journal of Pharmaceutical Sciences and Research 2011; 2(6): 1356-1377.
60. Nitta A, Fujii H, Sakami S, Nishimura Y, Ohyama T, Satoh M, Nakaki J, Satoh S, Inada C, Kozono H, Kumagai H, Shimamura M, Fukazawa T and Kawai H: (3R)-3-Amino-4-(2, 4, 5-triluorophenyl)-N-{4-[6-(2-methoxy) benzothiazol-2-yl] tetrahydropyran-4-yl}butanamide as a potent dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorganic & Medicinal Chemistry Letters 2008; 18: 5435-5438.
61. Paoli P, Vazquez GN, Rivera IL, Molina RV, Franco J, Andrade RO, Estrada-Soto S, Camici G, Coutino D, Ortiz I, Mayorga K and Diaz H: Synthesis, in vitro and computational studies of protein tyrosine phosphatase 1B inhibition of a small library of 2-arylsulfonylamino benzothiazoles with antihyperglycemic activity. Bioorganic & Medicinal Chemistry 2009; 17: 3332-3341.
62. Omar AMME, Aboulwafa OM, Issa DAE, El-Shokrofy MMS, Amr ME and El-Ashmawy IM: Design, facile synthesis and anthelmintic activity of new O-substituted 6-methoxybenzothiazole-2- carbamates. Med Chem Comm 2017; 8(7): 1440-1451.
63. Munirajasekhar D, Himaja M, Mali SV, Karigar A and Sikarwar M: Synthesis and Anthelmintic activity of 6-Substituted 2-Hydrazino-1, 3-Benzothiazoles. J Pharm Research 2011; 4(7): 2186-2187.
64. Suresh CH, Rao JV and Jayaveera KN: Anti-inflammatory activity of 3-(2-hydrazino benzothiazoles)-substituted indole-2-one. International Journal 2011; 1: 30-34.
65. Sreenivasa GM, Jayachandran E, Kumar R, Jaya and Vijay MMJ: Synthesis of Bioactive molecule fluorobenzothiazole comprising potent heterocyclic moieties for Anthelmentic activity. Archives of Pharmacal Research 2009; 1: 150-157.
66. Ghoneim KM, Essawi MYH, Mohamed MS and Kamal AM: Synthesis of 2-[(4-amino or 2,4-diaminophenyl) sulfonyl] derivatives of benzimidazole, benzothiazole and 6-methyluracil as potential antimicrobial agents. Indian J Chem 1998; 37B: 904.
67. Nagarajan S, Crescenzo G, Getman D, Lu H, Sikorski J, Walker J, McDonald J, Houseman K, Kocan G, Kishore N, Mehta P, Shippy C and Blystone L: Discovery of Novel Benzothiazolesulfonamides as potent inhibitors of HIV-1 Protease. Bioorganic & Medicinal Chemistry 2003; 11: 4769-4777.
68. Chi B, Jill C, David P, Song J, Choy W, Lambert P and Gagne D: Discovery of benzothiazole derivatives as efficacious and enterocyte-specific MTP inhibitors. Bioorg Med Chem Lett 2009; 19: 1416-20.
69. Sarkar S, Asim A, Siddiqui AA, Saha SJ, De R, Mazumder S, Banerjee C, Iqbal MS, Nag S, Adhikari S and Bandyopadhyaya U: Antimalarial activity of small-molecule benzothiazole hydrazones. Antimicrobial Agents and Chemotherapy 2016; 60(7): 4217-4228.
70. Bowyer PW, Gunaratne RS, Grainger M, Withers-martinez C, Wickramsinghe S, Tate EW, Leatherbarrow RJ, Brown KA, Holder AA and Smith DF: Molecules incorporating a benzothiazole core scaffold inhibit the N-myristoyltransferase of Plasmodium falciparum. Biochemical Journal 2007; 408: 173-180.
71. Hout S, Azas N, Darque A, Robin M, Giorgio C, Gasquet M, Galy J and David P: Activity of benzothiazoles and chemical derivatives on Plasmodium falciparum. Parasitology 2004; 129: 525-542.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE