About Authors:
Vinay Mishra, Shilpi Bhargava
Advance Institute of Biotech and Paramedical Sciences,
Kanpur
Introduction:
Delivering medicine to the general circulation through the skin is seen as a desirable alternative to taking it by mouth. Patientsoften forget to take their medicine, and even the most faithfullycompliant get tired of swallowing pills, especially if theymust take several each day. Additionally, bypassing the gastrointestinal(GI) tract would obviate the GI irritation that frequently occursand avoid partial first-pass inactivation by the liver. Further,steady absorption of drug over hours or days is usually preferableto the blood level spikes and troughs produced by oral dosageforms.1
From 1979, when the Food and Drug Administration approved the first transdermal drug delivery system (Transderm Scop® Patch), to the current transdermal delivery systems, there evolved a successful alternative to systemic drug delivery. Despite their relatively higher costs, transdermal delivery systems have proved advantageous for delivery of selected drugs, such as estrogens, testosterone, clonidine, nitroglycerin, scopolamine, fentanyl, and nicotine.
Transdermal therapeutic systems are defined as self contained, discrete dosages forms which when applied to the intact skin, deliver the drug, through the skin, at a controlled rate to the systemic circulation.Transdermal drug delivery systems, where applicable, offer several advantages over other conventional dosage forms.2
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1183
Advantages:
Using TDDS, it is possible to achieve the following advantages:
1. Avoidance of the 'first pass effect'.
2. A stable and controlled blood level.
3. Comparable characteristics with intravenous infusion.
4. Termination of further administration, if necessary.
5. Long-term duration (ranging from a few hours to one week).
6. No interference with gastric and intestinal fluids.
7. Administration of drugs with:
- A very short half-life
- Narrow therapeutic window
- Poor oral absorption
8. Improved patient compliance and reduced inter and intra-patient variability.
9. Self administration is possible.
10. Systems are non invasive.
Limitations:
Transdermal drug delivery systems have some limitations:
1. The drug must have some desirable physico-chemical properties for penetration through stratum corneum.
2. Skin irritation or contact dermatitis due to drug or exipients.
3. The barrier function of skin changes from one site to another on the same person, from person to person and with age. 6,8
Morphology Of skin:
Anatomically the skin has three major tissue layers- the epidermis, the dermis and the hypodermis.
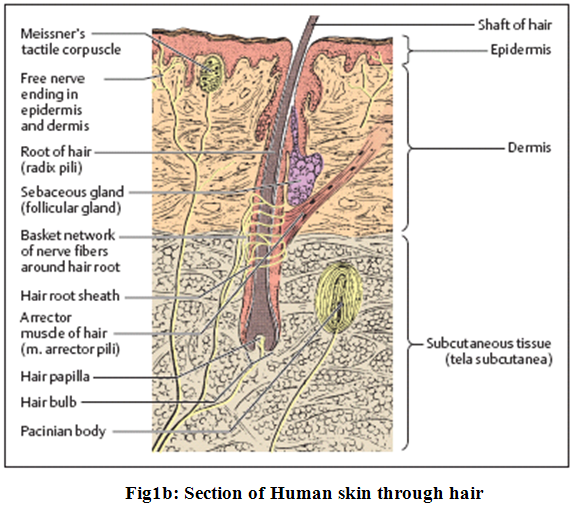
The epidermis with its epithelium forms the surface layer of the body surface. It is a stratified squamous keratinized (horny) epithelium, and is in most areas of the body 0.1−0.2 mm thick. The cells divide constantly in the lowest cellular layers (collectively called germinative stratum, stratum germinale), the basal and prickle (or spinous) cell layers (stratum basale and stratum spinosum) (Fig.1a and 1b).
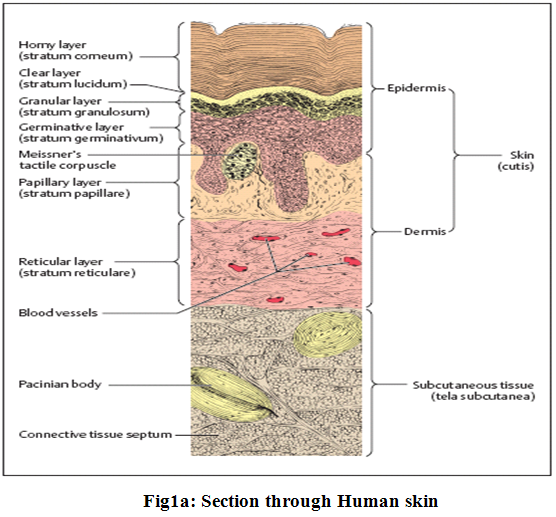
In this process, one daughter cell migrates to the surface, while the other divides again. As the cells migrate toward the surface to become cornified, they form granules (stratum granulosum).9
The major barrierwithin the skin is the stratum corneum, the top layer of theepidermis. The stratum corneum consists of keratinized, flattenedremnants of once actively dividing epidermal cells. Hygroscopic,but impermeable to water, it behaves as a tough, flexible membrane.The intercellular space is rich in lipids. The stratum corneumis about ten microns thick, but on the palms and soles it rangesup to 600 microns in thickness3.
[adsense:468x15:2204050025]
Percutaneous absorption and kinetics of transdermal permeation:
Percutaneous absorption involves passive diffusion of substances through the skin. The mechanism of permeation can involve passage through the epidermis itself (transepidermal absorption) or diffusion through shunt particularly hair follicles and eccrine glands (transfollicular or shunt pathway absorption).
(A)Transepidermal absorption:
Transepidermal pathway is principally responsible for diffusion across skin. Permeation by the transepidermal route first involves partitioning into stratum corneum than diffusion takes place across this tissue. Most substances diffused across the stratum corneum via the intercellular lipoidal route.
(B)Transfollicular (shunt pathway) absorption:
The skin’s appendages offer only secondary route for permeation. Sebaceous and eccrine glands are the only appendages which are seriously considered as shunts bypassing the statum corneum.The follicular route remains an important route for percutaneous absorption since the opening of follicular pore, where the hair shaft exits the skin, is relatively large and sebum aids in diffusion of penetrants.
Transdermal permeation of a drug involves the following steps:
1. Sorption by stratum corneum
2. Penetration of drug through viable epidermis
3. Uptake of the drug by cappillary network in the dermal papillary layer
This permeation can be possible only if the drug possesses certain physicochemical properties.The rate of permeation across the skin (dQ/dt) is given by-
dQ/dt=Ps(Cd-Cr)
where
Cd-the concentrations of skin penetrants in the donor compartment(surface of stratum corneum)"
Cr - the concentrations of skin penetrants in receptor compartment (body)
Ps - overall permeability coefficient of the skin tissues to the penetrant.
Ps=Ks.Dss / hs
where
Ks - partition coefficient
Dss - apparent diffusivity for steady state diffusion
hs - overall thickness of skin tissues.
A constant rate of drug permeation can be obtained only when Cd >> Cr i.e. the drug concentration at the surface of stratum corneum (Cd) is consistently and substantially greater than the drug concentration in the body-
dQ/dt = Ps . Cd
When Cd >> Cs then maximum rate of skin permeation (dQ/dt)m
(dQ/dt)m = Ps . Cs
The maximum rate of skin permeation depends on:
-The skin permeability coefficient (Ps) and
-Equilibrium solubility in stratum corneum (Cs).
Thus skin permeation appears to be stratum corneum limited.6
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Factors affecting transdermal permeability:
The factors influencing transdermal permeability of stratum corneum can be classified into 3 major categories:
1. Physicochemical properties of the penetrants
2. Physicochemical properties of the drug delivery systems
3. Physiological and pathological conditions of the skin
(A) Physicochemical properties of the penetrants:
1. Partition coefficient- Drugs possessing both water and lipid solubilities are favorably absorbed through skin. Transdermal permeability coefficient shows in linear dependency on partition coefficient. A lipid/water partition coefficient of 1 or greater is generally required for optimal transdermal permeability.
2. pH conditions- pH conditions of skin surface and in drug delivery systems affect the extent of dissociation of ionogenic drug molecules and their transdermal permeability.
3. Penetrant concentration- Transdermal permeability across mammalian skin is a passive diffusion process thus depends on the concentration of penetrant molecules on the surface layers of the skin.
(B) Physicochemical properties of drug delivery systems:
1.Release characteristics- Generally, the more easily the drug is released from the delivery system, the higher the rate of transdermal permeation .The mechanism of drug release depends on whethere the drug molecules are dissolved or suspended in the delivery system and on interfacial partition coefficient of the drug from delivery system to the skin tissue.
2. Composition of drug delivery systems-The composition of drug delivery system has a great influence on percutaneous absorption of a drug molecule. It may affect not only the rate of drug release but also the permeability of stratum corneum by means of hydration, mixing with skin lipids or other sorption promoting effects.
3. Enhancement of transdermal permeation- Transdermal permeation of drugs can be improved by the addition of a sorption or permeation promoter in the drug delivery system
(a) Organic solvents as permeation promotor-
e.g. Dimethylsulfoxide(DMSO), Dimethylacetamide, Dimethylformide, Ethylene glycol, Polyethylene glycol, Ethanol
(b) Surface active agent as permeation promoter- The anionic surfactants are most effective permeation promoters. e.g. Sodium lauryl sulfate, Sodium dioctyl sulfosuccinate.
(C) Physiological and pathological conditions of the skin:
1. Reservoir effect of horny layer- The horny layer especially its deeper layer can act as a depot or reservoir and modify transdermal permeation characteristics of some drug.
2. Lipid film- Lipid film on skin surface, formed by product of the excretion of sebaceous gland and epidermal cell lipid maintains the barrier function of stratum corneum.
3. Skin hydration- Hydration of stratum corneum can enhance the permeability of the skin by as much as eight fold.
4. Skin temperature- A ten fold in the skin permeation was raised from 100 to 370 C of acetyl salicylic acid and glucosteroids was noticed when the environmental temperature7.
Classification of transdermal drug delivery systems:
Transdermal drug delivery systems generally fall into the following subcategories:
(1) Polymer membrane permeation-controlled
(2) Polymer matrix diffusion-controlled
(3) Drug reservoir gradient-controlled
(4) Micro reservoir dissolution-controlled
- Liquid-filled laminate structure
- Peripheral-adhesive laminate structure
- Solid-state laminate structure
- Sub-classes of the above
In terms of the drug release mechanisms, TDDS can be divided into six categories:
(1) Solution in matrix
(2) Suspension in continuous matrix
(3) Suspension in porous matrix
(4) Solution upstream of membrane
(5) Suspension upstream of membrane
(6) Laminated membrane downstream
However, TDDS are most commonly classified into two groups:
(A) Matrix patches-
In the matrix system, the inert polymer matrix binds with the drug and controls its release from the device (e.g., Nitro-Dur® Patch, Nicotrol® Patch [OTC], and Vivelle® Transdermal).
(B) Liquid reservoir patches-
In the reservoir system, the polymer matrix does not control drug release. Instead, a rate-controlling membrane present between the drug matrix and the adhesive layer provides the rate-limiting barrier for drug release from the device. (e.g. Transderm-Nitro® Patch, Transderm Scop® Patch, Catapres-TTS® Transdermal, Estraderm® Transdermal, Duragesic® Transdermal, Nicoderm® CQ Patch, and Androderm® Transdermal System).4,8
Basic Components of TDDS:
Both matrix patches and liquid reservoir patches consist of several components. Some of these are similar in both classes, while others are class-specific. Those common to both include:
(1) Backing films
(2) Release liners
(3) Pressure-sensitive adhesives
(4) Active ingredient(s)
(5) Permeation enhancers
(6) Other additives
(7) Micro porous or semi-permeable membranes
(8) Pouching materials
Although the pouching materials do not represent a direct component of patches, it is included because, firstly, a patch is never fully isolated from its pouching material. Secondly, it plays a critical role in the patch's stability, and finally, it protects children (in cases of Nicotine and Fentanyl) from a very strong and dangerous active.
(1) Backing Films-
Backing films play a critical role in the TDDS (as long as they are packaged in their pouch), as well as during the use of the system. The role of such a film is to protect the active layer and safeguard the stability of the system, and to affect skin permeation and tolerance, depending on occlusion or breathability. Because of the huge variety of ingredients, the release liner must be fully inert to the ingredients in order to avoid any type of incompatibility. It must also be flexible, comfortable and must present good affinity with the adhesive, as well as excellent printability. The most common materials used as release liners are polypropylene, polyethylene (both high and low density), saran, polyesters, PVC and nylon.
(2) Release Liners-
Generally, a release liner is a film covered with an anti-adherent coating. The role of the release liner is to protect the system as long as it is in the package, and it is removed just before the adhesion of the TDDS to the skin. Release liners play a crucial role in the stability of the product and in its safe and functional use. The release liner must therefore be chosen very carefully.
An incorrect release liner does not permit the easy release of the patch, and can interfere with the active(s) or other components, thereby reducing its shelf life. The most common films used as release liners are paper-based, plastic film-based and composite films. The two major classes of coating are silicones and fluoro-polymers.
(3) Pressure-Sensitive Adhesives-
For both classes of TDDS, pressure-sensitive adhesives (PSAs) play a major role, serving as the matrix that carries everything active (such as additives and permeation enhancers) and the means for making the patch stick to the skin. There are three major families of PSAs: rubber-based PSAs, acrylic PSAs in the formof acrylic solutions, emulsion polymers or hot melts, and silicon PSAs. For each family of adhesives there are several sub-categories that give the required flexibility to the formulator.
Each active is different and the choice of adhesives is critical for the success of the final product. There are several examples (Table 1), such as:
Acrylics:
1. With or without functional groups
2. Cross-linked or not
Solutions, hot melts or emulsions
Silicon-Based Adhesives:
1. Standard
2. Amine-compatible
Rubbers with different:
1. Tackifiers
2. Cross-linkers
3. Stabilisers and plasticizers
(4) Penetration Enhancers-
This term refers to an entire family of chemically different substances that all share a common characteristic - they facilitate the permeation of the actives through the skin, increasing the permeation rate by several times. This is very important with respect to the feasibility of a system, because most of the actives do not enter the skin in the required dosage from a relatively small area. Sometimes a combination of ingredients is needed to create the correct enhancement effect.
|
Table 1: Pressure-Sensitive Adhesives in TDDS |
|||
|
|
Advantages |
Disadvantages |
|
|
Rubber Adhesives |
Good adherence to low and high energy surfaces |
Poor ageing |
|
|
Relatively inert adhesives |
Low shear and irradiation resistance |
||
|
High initial adhesion |
M.W. variability |
||
|
Useful in broad temperature range
|
Low tack and adhesion without additives |
||
|
Flexibility |
|
||
|
Low cost |
|
||
|
Acrylic Adhesives |
Good UV, solvent and hydrolysis resistance |
Moderate cost |
|
|
Excellent adhesion build-up
|
Fair initial adhesion |
||
|
Good shear strength |
Poor creep |
||
|
Easy to apply |
|
||
|
Good service life |
|
||
|
Silicon-Based PSAs |
Chemical and biological inertness |
Highest cost |
|
|
Extremely low toxicity, sensitisation and irritation
|
The need for expensive release liners
|
||
|
Retaining of mechanical and physiochemical properties on the skin |
No aggressive behaviour |
||
(5) Micro porous or Semi-Permeable Membranes-
The porous membrane is a special kind of plastic film which is used in all liquid reservoir patches and in some matrix patches. Its role is to limit the flow of the semi-solid content from the liquid reservoir, and/or to act as a rate-limiting membrane for both liquid reservoir and matrix systems. The function depends on the design of the specific system, the size of the active component and the need to have a rate-limiting factor in order to satisfy the release and absorption characteristics of the system. The chemical composition of these membranes is a key factor in their permeability.
There are two types of porous membranes-
A. Ethylene Vinyl Acetate Membranes (EVA)
B. Micro porous Polyethylene Membranes
(6) Pouching Materials-
Most patches for use in a TDDS are packaged as unit doses in sealed pouches. The pouching material is therefore critical to the stability and integrity of the product. When there are two films with similar positive characteristics, the one with the lower cost, better machinability and better printability will be chosen.
There are three main layers in the composite materials used for pouches: the internal plastic heat sealable layer, the aluminium foil layer and the external printable layer. If the film is a lamination, an adhesive is used to keep the layers together. Alternatively, an extrusion method can be used if it is suitable.
a. Heat Sealable Layer:
This layer plays an important role in the machinability, stability and protection of the product. Several plastic films or coatings can be used for its formation, including polyethylene, surlyn and other similar heat sealable materials.
b. Aluminium Foil Layer:
This layer plays an important role in protecting the product from light and oxygen. In 'ideal' conditions the foil needs to have a thickness of more than 1mil or 25 micrometers to be a real barrier. If any less than this thickness level is used, there will always be pinholes reducing the barrier properties.
c. External Layer:
The external layer of a composite film or laminate is used to achieve a better 'finishing' and printing quality. It can also compensate for a lack of stability as it acts synergistically with the aluminium foil. Paper or polyester film is used for this role, but the polyester film creates a better-looking pouch and a better barrier.6,8
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Approaches Used In Development of tDDS:
4 different approaches have been utilized to obtain transdermal drug delivery systems:
1. Membrane permeation controlled systems:
In this type of system the drug reservoir is totally encapsulated in a shallow compartment moulded from a drug impermeable metallic plastic laminated and rate controlling polymeric membrane which may be micro porous or nonporous e.g. ethylene vinyl acetate(EVA) copolymer , with a defined drug permeability property (Fig.2).
The constant release of the drug is the major advantage of membrane permeation controlled transdermal system. e.g.
1 Nitroglycerin releasing transdermal system (Transderm- Nitro)
2 Scopolamine releasing transdermal system (Transderm-Scop)
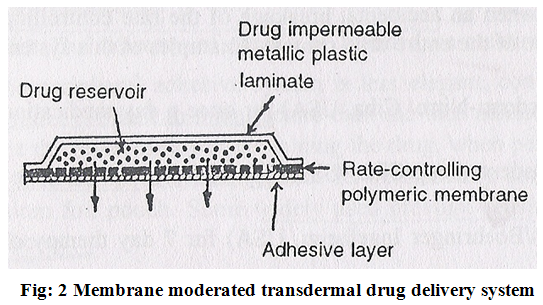
The intrinsic rate of drug release from these drug delivery systems is defined by-
dQ/dt = CR / (1/Pm + 1/Pa)
where
CR- the drug concentration in the reservoir compartment
Pa-the permeability coefficient of the adhesive layer
Pm -the permeability coefficient of rate controlling membrane
2. Adhesive dispersion type systems:
It is a simplified form of membrane permeation controlled system. Drug reservoir is formulated by directly dispersing the drug in adhesion polymer and then spreading the medicated adhesive, by solvent casting or hot melt, on to a flat sheet of drug impermeable metallic plastic backing to form a thin drug reservoir layer. e.g. Isosorbide dinitrate transdermal therapeutic system (Fig.3).
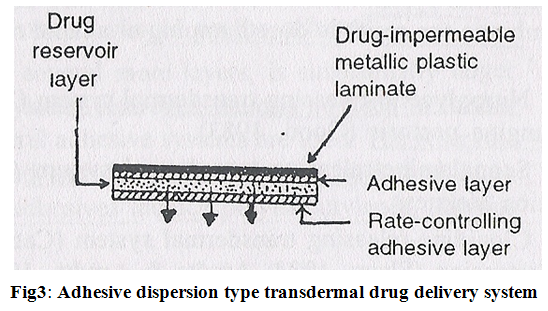
The rate of drug release in this system is-
dQ / dt = Ka/r.Da .CR
ha
where
Ka/r – partition coefficient for the interfacial partitioning of the drug from the reservoir layer to adhesive layer.
3. Matrix diffusion controlled systems:
In this drug reservoir is prepared by homogeneously dispersing drug particles into hydrophilic or lipophilic polymer matrix. The resultant medicated polymer is then moulded into a medicated disc with a defined surface area and controlled thickness.
This drug reservoir containing polymer disc is then pasted on to an occlusive base plate in a compartment fabricated from a drug impermeable plastic backing.
The adhesive polymer is then spread along the circumference to form a strip of adhesive rim around the medicated disc (Fig.4).
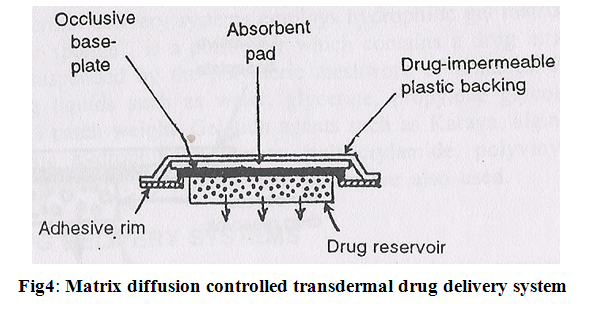 The rate of drug release from this type of system is –
The rate of drug release from this type of system is –
dQ / dt = {A Cp.Dp/2t}1/2
Where
A- initial drug loading dose dispersed in polymer matrix
Cp-solubility of drug in polymer
Dp-diffusivity of drug in polymer
4. Micro reservoir type or microsealed dissolution controlled system:
It is a combination of reservoir and matrix diffusion type drug delivery system. The drug reservoir is formed by first suspending the drug solids in an aqueous solution of a water soluble liquid polymer and then dispersing the drug suspension homogeneously in lipophilic polymer viz. silicone elastomers by high energy dispersion technique to form several discrete, unleachable microscopic spheres of drug reservoirs (Fig.5)
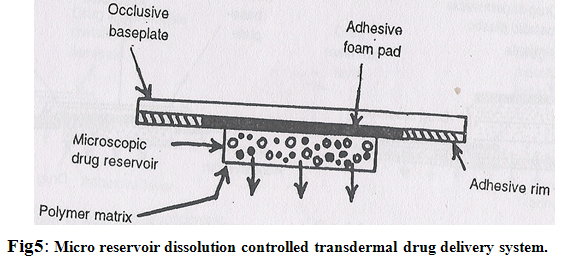
A transdermal therapeutic system is produced by positioning the medicated disc at the centre and surrounding it with an adhesive rim. The micro reservoir system has been claimed to follow the zero order release of drugs without the danger of dose dumping.6
Examples of transdermal drug delivery systems:
1. Transdermal scopolamine-
The transderm-Scop system is a circular flat patch 0.2mm thick and 2.5 cm2 in area. It is a four layer system. It contains 1.5 mg of scopolamine. The patch is worn in a hairless area behind the ear.
e.g. Transderm-Scop
2.Transdermal nitroglycerine-
Drug reservoir = Nitroglycerine-lactose in a silicone medical fluid. Constructed from a drug-impermeable plastic /metal laminate. Rate-controlling microporous membrane of ethylene vinyl acetate copolymer. Thin layer of pressure sensitive silicone adhesive polymer coated on the surface membrane to achieve contact with the skin engineered to deliver nitro at dosage of 0.5 mg/ cm2/day to provide daily relief from angina attacks.
e.g. Transderm-Nitrosystem, Nitro-Dur, Deponit and Minitran
3. Transdermal clonidine-
Clonidine transdermal therapeutic system provides controlled release of clonidine for seven days. This is applied to hairless area of upper arm or torso. It is four layered patch.
e.g. Catapres –TTS
4. Transdermal nicotine-
Transdermal therapeutic systems providing continuous release and systemic delivery of nicotine as an aid in smoking cessation programs. The commercially available patches contain 7 to 22 mg nicotine.
e.g. NicoDerm-CQ, Nicotrol, Habitrol
5. Transdermal estradiol-
It is designed to release 17β-estradiol. Patch is generally applied twice weekly over a cycle of three weeks with dosage frequency adjusted as required. It is four layered patch.
e.g. Estraderm, Vivelle, Climara
6. Transdermal Fentanyl-
This patch provides continuously 72 hours systemic delivery of fentanyl, a potent opioid analgesic. It is four layered patch
e.g. Duragesic. 5
Evaluation of Transdermal Drug deliverySystems:
1. Evaluation of adhesive-
Pressure sensitive adhesive are evaluated for following properties:
(A) Peel adhesion properties;
Peel adhesion force required to remove adhesive coating from a test substrate. Peel adhesion properties are affected by the molecular weight of adhesive polymer, the type and amount of additives and polymer composition (Fig.6).
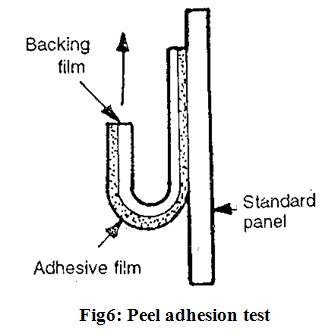
It is tested by measuring the force required to pull a single coated tape, applied to a substrate, at an 180?angle. No residue on the substrate indicates adhesive failure which is desirable for transdermal devices.
(B) Tack properties;
Tack is ability of polymer to adhere to a substrate with little contact pressure. It is important in transdermal devices which are applied with finger pressure. Tests for tack include;
1. Thumb tack test-
This is a subjective test in which evaluation is done by pressing the thumb briefly into the adhesive.
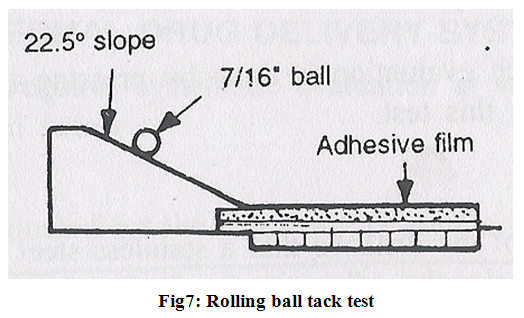
2. Rolling ball tack test-
it involves measurement of the distance that a stainless steel ball travels along an upward facing adhesive .The less tacky the adhesive, the farther the ball will travel (Fig.7).
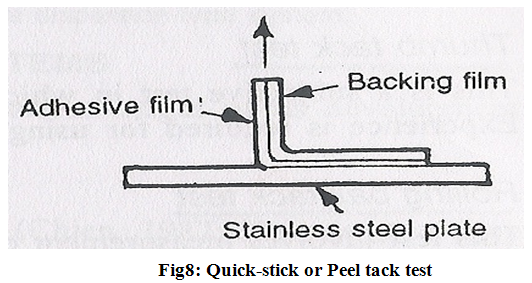
3. Quick-stick or Peel tack test-
The peel force required to break the bond between an adhesive and substrate is measured by pulling the tape away from substrate at 90? at a speed of 12 inch/min. the force is recorded as tack value and is expressed in ounce (or grams) per inch width with higher values indicating increasing tack (Fig.8).
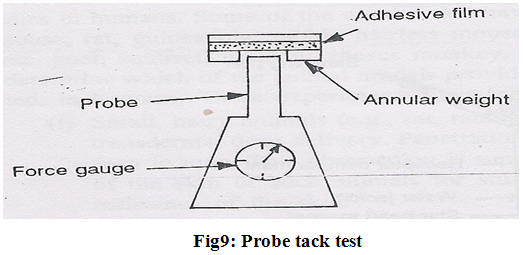
4. Probe tack test–
The force required the pull a probe away from an adhesive at a fixed rate is recorded as tack (expressed in grams) (Fig.9).
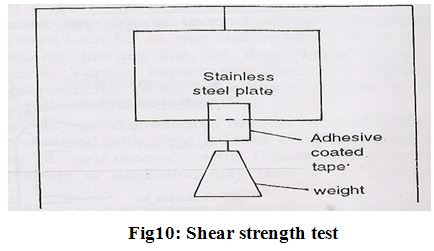
(C) Shear strength properties;
shear strength is measurement of the cohesive strength of an adhesive polymer. Shear strength or creep resistance is determined by measuring the time it takes to pull an adhesive coated tape off a stainless steel plate when a specified weight is hung from the tape which pulls the tape in a direction parallel to the plate (Fig.10).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2.In- vitro drug release evaluation:
In vitro studies can help in investigating the mechanism of skin permeation of the drug before it can be developed into a transdermal therapeutic system. In in-vitro studies, excised skin is mounted on skin permeation cells. The non availability of human cadaver skin which is most logical choice for in-vitro studies, has led investigation of other animal skin. Hairless mouse skin is generally favoured.
Ideally an in-vitro system should be designed in such a way that the intrinsic rate of release or permeation which is theoretically independent of in-vitro design can be accurately determined.
In-vitro membrane permeation apparatus includes Valia-Chien (V-C) cell, Ghannam-Chien (G-C) membrane permeation cell, Jhawer –Lord (J-L) rotating disc system, Franz diffusion cell and Keshary-Chien (K-C) cell.
The most widely used cell is Franz diffusion cell and Keshary-Chien (K-C) cell. K-C Cell (modified version of Franz diffusion cell) has an effective receptor volume of 12 ml and skin surface area of 3.14 cm.2 .The receptor solution is stirred by a star- head magnet rotating at a constant speed of 600 rpm driven by a 3-W synchronous motor.
3. Effect of skin uptake and metabolism:
Depending on the pharmacological activity of the metabolite formed, the metabolic reaction occurring in the skin could affect the therapeutic activity. Permeation and bioavailability of a drug at the site of application of prerequisites to any possible metabolic reactions in the skin and the factors affecting the skin permeation of a drug would also influence the metabolism of a drug in skin.
For studying in-vitro skin uptake and metabolism of drug, the stripped skin was mounted between the two compartments of each V-C permeation cell. The stratum corneum or dermis is faced the drug solution and other side of the skin was protected with impermeable aluminum foil .The compartment was filled with saturated solution of drug in normal saline and was maintained isothermally at 37ºC.
4. In-vivo evaluation:
In-vivo evaluation of transdermal drug delivery systems can be carried out using-
A. Animal models
B. Human volunteers
C. Biophysical models
(A) Animal models: For In-vivo testing mouse, rat, guinea pig, rabbit, hairless dog and cat are used. The rhesus monkey is the most reliable model for in vivo evaluation of transdermal drug delivery in man.
(B) Human model: The final stage in the development of transdermal device involves collections of pharmacokinetic and pharmacodynamic data following application of the device to the human volunteers. To overcome the limitations inherent in this method two refinements are made-
1. Reservoir technique (using radiolabelled compound)
2. Mass balance technique
(C) Biophysical models: These models are based on steady state mass balance equation, solution of Fick’s law of diffusion for the device, stratum corneum and viable epidermis as well as linear kinetics.
5. Cutaneous toxicological evaluations:
A. Contact dermatitis: contact dermatitis can be either contact irritant dermatitis or contact allergic dermatitis.
B. Growth of micro-organism: Bacteria, yeast and fungi may proliferate under occlusive dressing due to favourable factors like increased temperature, hydration, pH etc. The growth of microorganism can be evaluated by quantitative bacteriological cultures of skin site before and after use transdermal system using nonionic detergent.6
Patient Counseling Issues Related to TDDS:
a. The patch should be applied to a clean, dry, non-hairy area of the skin with reasonable and uniform pressure. Oily, inflamed, broken, or calloused skin should not be used
b. Percutaneous absorption may vary depending upon site of patch application. Regions of the skin with thick epidermal layers, such as palms and soles, should be avoided. The post-auricular region is best suited for small-sized patches, as it is richly innervated by blood vessels. Most often, larger patches are applied on the forearm, inner thigh, lower back, or chest.
c. The patch should be worn only for the designated period of time and not any longer, as drug release rate is not ensured beyond the designated time.
d. For repeated use of transdermal patches, application sites should be rotated.
e. Transdermal patches should be stored in original sealed pouches until the time of use. If the package is damaged, it may lead to drug loss or altered physicochemical properties of one or more components.
f.Handling of the patch during removal from its package and its application is especially important. Care should be taken to avoid touching or damaging the adhesive surface (which may contain drug) after removal of the release liner.
g. When applying a patch to the skin, it should be firmly pressed against the skin with the heel of the hand for about 10 seconds to ensure uniform contact and adhesion. 4, 5
The Future of Transdermal Therapy:
Ten years ago, the nicotine patch had revolutionized smokingcessation; patients were being treated with nitroglycerin forangina, clonidine for hypertension, scopolamine for motion sickness,and estradiol for estrogen deficiency, all through patches.During the past decade biotech products have come into theirown, but transdermals have essentially remained static. Thenumber of drugs formulated in patches has hardly increased,and there has been little change in the composition of the patchsystems. Modifications have been mostly limited to refinementsof the materials used.
A. Molecular Absorption Enhancement:
Considerable research has been done on absorption enhancers,compounds that promote the passage of drugs through the stratumcorneum. Terpene derivatives as well as certain phenols seemto improve transdermal absorption. For example, linalool, alphaterpineol, and carvacrol were studied in conjunction with haloperidol(a commonly prescribed neuroleptic drug). All three enhancedhaloperidol absorption, but only linalool increased it to atherapeutic level. Limonene, menthone, and eugenol werefound to enhance transdermal absorption of tamoxifen. Phloretin,a polyphenol, enhanced the absorption of lignocaine.
B.Absorption Enhancement by Energy Input:
The above are potential adjuvants to the existing "passive"transdermal systems. Also under study is the possibility ofactive transfer of drugs through the skin by the action of electricalor other forms of energy. The most research has been devotedto iontophoresis, sonophoresis and electroporation have beenless well studied.
Iontophoresis- It is a method of transferring substances acrossthe skin by applying an electrical potential difference. Itpromotes the transfer of charged ionic drugs and possibly highmolecular weight substances such as peptides.
Electric currentis applied through two electrodes, placed on the patient’sskin. The first, or donor, electrode (cathode) delivers thenegatively charged therapeutic agent (e.g., an organic acid),whereas the second, or receptor, electrode (anode) serves toclose the circuit. This setup is named cathodal iontophoresis.For positively charged drugs (e.g., amines or peptides), thecell arrangement is reversed (anodal iontophoresis). The silver(anode) and silver chloride (cathode) electrode system—utilizedin both types of iontophoresis––is favored largelybecause it does not affect the drug solution to the extent thatother electrode systems can. Current commercial applications of iontophoresis include intradermaladministration of lidocaine as a local anesthetic and dexamethasonefor local inflammation.
Sonophoresis- Low frequency ultrasound treatment was reportedto enhance absorption of mannitol, which was used as a modelfor highly hydrophilic drugs.
Electroporation- It is a technique that delivers high voltage pulsesof micro-to-millisecond duration to the skin, causing transientchanges in cell membranes or lipid bilayers. It is hypothesizedthat pretreatment of the skin in this way would enable the passageof large, polar molecules such as heparin and peptides.2,3
References:
1. Gennaro,AR, Remington’s Practice of Pharmacy; 20th ed, 2000;Lippincott Williams & Wilkins,New York, 836
2. Swarbrick, J. and Boylan, J.C., Eds. Encyclopedia of Pharmaceutical Technology, 2nd ed. New York: Marcel Dekker, Inc. 2002,1573–1587
3. Scheindlin S., Transdermal Drug Delivery: PAST, PRESENT, FUTURE, Molecular Interventions, American Society for Pharmacology and ExperimentalTherapeutics,4:308-312,(2004)
4. Mehta, Ratna, Topical and Transdermal Drug Delivery: What a Pharmacist Needs to Know; ACPE ID number 221-146-04-054-H01
5. Ansel HC, Allen LV Jr., Popovich NG, Pharmaceutical Dosage Forms and Drug Delivery Systems; 7th ed., 1999.Lippincott Williams and Wilkins; New York; 263-277
6. Jain N.K.; Controlled and Novel Drug Delivery; 1st ed., 1997;CBS Publishers & Distributors, New Delhi;100-127
7. Chien, Y. W., Novel Drug Delivery Systems; 2nd ed., 1992;Marcel Dekker, Inc., New York;185-190
8. Spyridon A. Fotinos, An Introduction to Transdermal Drug Delivery; 1-15
9. Faller, The Human Body; 2004; Lippincott Williams and Wilkins; New York; 665-669
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE










