About Author:
Vivek P. Chavda
Department of Pharmaceutics,
B.K. Mody Government Pharmacy College,
Rajkot – 360003, Gujarat (India)
vivek7chavda@gmail.com
Introduction
Thermal method of analysis are group of techniques in which changes in physical and /or chemical properties of a substance are measured as a function of temperature, while substance is subjected to controlled temperature programmes.[1]Thermal analysis and calorimetric methods have demonstrated a wide array of applications in the Preformulation, and formulation development. These techniques are critical in physical–chemical screening of early discovery leads, during salt form screening, and in the characterization of polymorphs to determine the thermodynamic relationships between the various crystal forms.[2]
REFERENCE ID: PHARMATUTOR-ART-1872
Figure 1: Thermal Analysis in Preformulation study
Preformulation involves the application of biopharmaceutical principles to the physicochemical parameters of drug substance are characterized with the goal of designing optimum drug delivery system. The wide range of measurements possible provide fundamental information on the material properties of the system under test, so thermal analysis has found increasing use both in basic characterization of materials and in a wide range of applications in research, development and quality control in industry and academia. Thermal analytical methods can measure the following physical properties; weight loss on drying, enthalpy, Glass transition temperature, gas evolution, electrical conductivity, optical characteristic, magnetic properties, changes in form in dimension and viscoelastic properties of substance.[3]Simultaneous Thermal Analysis (STA) generally refers to the simultaneous application of Thermogravimetric (TGA) and differential scanning calorimetry (DSC) to one and the same sample in a single instrument. The test conditions are perfectly identical for the TGA and DSC signals (same atmosphere, gas flow rate, vapor pressure of the sample, heating rate, thermal contact to the sample crucible and sensor, radiation effect, etc.). The information gathered can even be enhanced by coupling the STA instrument to an Evolved Gas Analyzer (EGA) like Fourier transform infrared spectroscopy (FTIR) or mass spectrometry (MS). [4]DSC, TG/DTA and TG/DTA-IR are often used for characterization of pharmaceuticals. DSC, alone or in combination with hot-stage microscopy, is able to differentiate between different polymorphic structures and, by using different heating rates, can investigate the transformations which occur during the polymorphic transformation. By using appropriate heating rates, polymorphic purity can be determined, and can involve heating rates up to 750°C/min. TGA is often used to measure residual solvents and moisture, but can also be used to determine solubility of pharma materials in solvents. Analysis of pharma materials is probably the largest area of application for thermal analysis.
Classification of Thermal Analytical Techniques
A detailed classification on thermal analytical techniques is shown in the Table 1 which is adopted from chatwal et al.,(2007)
Table 1: Different thermal analysis techniques
|
Physical properties |
Name of technique |
Instrument used |
|
Mass |
Thermogravimetry |
Thermo balance |
|
Evolved gas analysis |
Evolved gas detector |
|
|
Temperature |
Differential thermal analysis(DTA) |
DTA apparatus |
|
Enthalpy |
Differential scanning calorimetry(DSC) |
Differential calorimeter |
|
Mechanical properties (dimension, viscoelastic properties) |
Thermo mechanical analysis |
TMA apparatus |
|
Thermodilatometry |
Dilatometer |
|
|
Acoustic characteristic |
Thermo acoustimetry |
|
|
Thermosonimetry |
||
|
Optical characteristic |
Thermoptometry |
|
|
Magnetic susceptibility |
Thermomagnetometry |
|
|
Electrical resistance or conduction |
Thermoelectrometry |
|
Various hyphenated technique are utilized now a days: Like for better interpretations TG-DTA and TG-DSC while for identification of gas involved in thermal analysis TG-IR, TG-GC-MS and TG-MS are used. Apart from that DSC-THERMOMICROSCOPY, DSC-FTIR, DSC-TEM and DSC-XRD were explored in this field.
Innovation in thermal analysis
1. Multielemental scanning thermal analysis(MESTA)
Identification and characterization of solid samples has been relatively difficult due to the limited separation techniques available. MESTA is the method for the identification & characterization of solid substance which is simple, rapid and sensitive alternative for routine examination of solid sample. Volatile components in the sample are carried to a high temp combustion tube where the C, N, S are oxidized to their respective oxides & detected by the detector.[5]
2. Micro thermal analysis
Micro thermal analysis is a relatively new technique that combines the resolution of an Atomic force microscope (AFM) with thermal conductivity measurement. MTA is useful for polymorph analysis. In this technique thermal conductivity is measured as a function of temp. MTA used for identification of components in compressed tablet and Analysis of tablet coats.Current pharmaceutical applications are then discussed; these include the identification of components in compressed tablets, the characterization of drug-loaded polylactic acid microspheres, the analysis of tablet coats, and the identification of amorphous and crystalline regions in semicrystalline samples.[6]
3. Modulated DSC
It is also called as oscillating DSC.Advantage of modulated DSC over traditional DSC method includesa. increased sensitivity, b. increased resolution, c. Separation of complex transitionsand d. Measurement of crystallinity.Thermal behavior of angiotensin II type 1 (AT(1)) receptor antagonist, Valsartan (VAL), was examined employing thermogravimetric analysis (TGA), standard differential scanning calorimetry (DSC) and temperature-modulated differential scanning calorimetry (TMDSC).TMDSC curve shows the glass transition process occurring at around 74°C. Results from standard DSC and TMDSC of Valsartan were compared over the whole range of temperature.[7]
4. Dynamic mechanical analysis
Here the mechanical response (Dynamic Modulus and /or damping) of a sample is measured as it is deformed under oscillating load against temp/time.The spatial variations in mechanical behavior of resin-infiltrated dentin using nanoscopic Dynamic Mechanical Analysis (DMA) was studied.In the hydrated state the apparent complex, storage and loss moduli for the resin-infiltrated dentin samples were 3.5±0.3GPa, 3.4±0.2GPa and 0.9±0.3GPa, respectively. Those values for the resin adhesive control were 2.7±0.3GPa, 2.7±0.3GPa and 0.2±0.02GPa, respectively. Viscoelastic deformation of the resin-infiltrated collagen exceeded that occurring in regions of uniform resin adhesive. Though dehydration resulted in a significant increase in both the complex and storage moduli of the macro hybrid layer, the largest changes occurred to the resin adhesive.[8]
5. Fast scan DSC
Conventional rate Differential Scanning Calorimetry (DSC) has been used for many years as a tool in the analysis of pharmaceutical materials. In recent years an extension of the technique to include fast heating and cooling rates has become more prevalent. Broadly termed Fast-Scan DSC, this review examines the current applications of this technique to the characterization and selection of pharmaceutical materials. Fast scan DSCdistinguishes melting, melting degradation, sublimation & thermal stability of drug.Useful for stability study where stability of drugs is based on a comparison of their thermal properties at various heating rates.Its increasing use encompasses the characterization of amorphousness in crystalline materials, the characterization of polymorphs and polymorphic transitions, the solubility of drugs in polymers, and characterization of dosage forms. Notwithstanding the advantages of analytical speed in analytical turnover, the review emphasizes the advantages of Fast-Scan DSC in its sensitivity which allows the separation of overlapping thermal events, the reduction it provides in degradation during the scanning process and its role in determining solubility in waxy and polymeric based systems.[9]
6. Optothermal Transient Emission Radiometry (OTTER)
In optothermal transient emission radiometry a sample material is irradiated (excited) with optical wavelength radiation in the form of short-duration pulses and the thermal emission transient observed by means of a wide-band infrared detector.This technique is suitable for the in situ non-destructive study of surfaces, and has been used to measure the thermal diffusivity of polyester films and pigments. More recently OTTER has been extended to the in vivo study of water concentration gradients in the stratum corneum.[10]
7. Specific Heat Spectroscopy
Specific heat spectroscopy (SHS) is a non-adiabatic technique used to measure the frequency-dependent heat capacity of organic materials near the glass transition over a wide frequency range. Using the fact that molecular relaxation times increase rapidly as the glass transition is approached from T > Tg , the dynamics of the liquid phase (and not the glassy phase) can be studied.[11]
8. Thermally Stimulated Current (TSC)
Thermally stimulated current (TSC) spectroscopy is used to characterize the relaxation processes and structural transitions occurring in samples that have been polarized at a temperature greater than the temperature where molecular motion in the sample is enhanced and subsequently quenched so that the high mobility state is frozen. On heating the sample at a controlled rate, depolarization of the polymer electrets (molecular or ionic dipoles, trapped electrons, mobile ions) occurs and the oriented dipoles, frozen in the quenched sample, relax to a state of thermal equilibrium. This relaxation process is observed as a depolarization current, which is typically of the order of picoamperes, and is referred to as the thermally stimulated current.[10]
Application of thermal analysis in Preformulation
1. Characterization of hydrates and solvates
Preformulation studies are commenced to identify the ability of drug to take up water and characterize the state of this water because this information is relevant in developing strategy for the process and storage of dosage form. TGA provides an important tool to characterize & quantify the moisture content in pharmaceutical material.[12]Presence of moisture & amount of moisture determines the stability of the sample. Two extremely water-soluble substances (flupentixol.2HCl and Lu 25–109, water solubility over 1000 mg/ml) and a hydrophobic substance (sertindole, approximately 10 μg/ml) were examined for critical moisture content by microcalorimetry. The values determined by isothermal microcalorimetry showed results similar to various weighing methods. This is known as characterization of hygroscopic property of API.
2. Study of polymer
Polymers represent another large area in which thermal analysis finds strong applications. Thermoplastic polymers are commonly found in everyday packaging and household items, but for the analysis of the raw materials, effects of the many additive used (including stabilizers and colors) and fine-tuning of the molding or extrusion processing used can be achieved by Thermal analysis. Thermogravimetric methods are largely limited to decomposition & oxidation reaction & to such physical process like vaporization, sublimation, desorption. Most important qualitative application of thermogravimetric methods are found in study of polymers. Thermogram provides information about decomposition mechanism for various polymeric preparation. In addition, the decomposition patterns are characteristic for each kind of polymer& in some case can be used for identification purpose.[13]
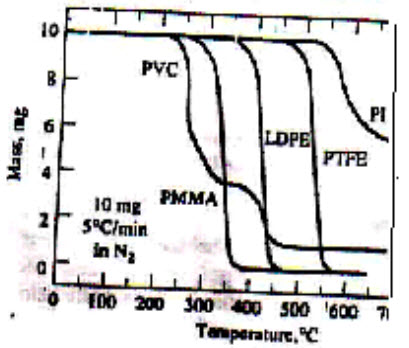
Figure 2: Qualitative analysis of polymers by TGA[1]
Thermogram is also used for quantitative analysis of a polymeric material. Polyethylene mixed with fine carbon black to inhibit degradation from exposure to sunlight. This analysis would be difficult by most other method.[1]
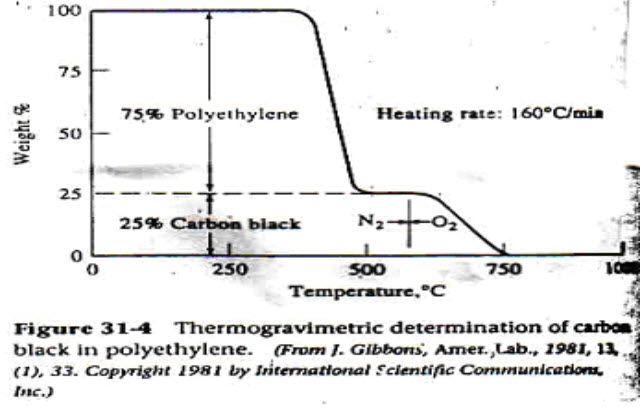
Figure 3: Quantitative polymer analysis by TGA[1]
The deterioration of polymeric materials is often caused by oxidation and typically manifested by gradually increasing yellowing and embrittlement. This cause instability of polymers which can be efficiently detected by microcalorimeter. Example: Study of oxidation of polyamide 6 film.
3. Detection of impurity
Thermal analytical system can be used for detection of impurities in pharmaceutical ingredient by recording TG Thermogram & DTA or DSC curves. This curves could be then be compared with the curve of reference standard. Any abnormal mass changes on TG curve & irregular endotherm or exotherm peaks on DTA or DSC curve would indicate the presence of impurity. A sharp melting endotherm indicates the relative purity whereas broad asymmetric curve suggest impurity. The presence of minute amount of substance broadens its melting range & lowers its melting point. Compare to other thermal methods, DSC is best method for detection of impurity. Eg. DSC of phenacetin.[14]
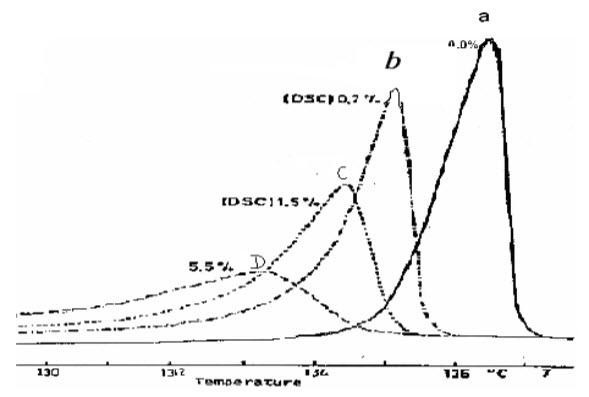
Figure 4: Broadening of Melting point curve of phenacetin due to impurities
4. Study of polymorphism
Polymorphism is the tendency of a substance to crystallize in different crystalline modification. The all thermodynamic parameter in the polymorphism substance is different like melting, sublimation temperature, kinetics, stability, solubility, heat capacity, crystal hardness & shape which are extremely important for the dosages form. During Preformulation, it is important to identify the polymorph that are stable & also important to determine whether polymorphic transition are possible within the temperature range used for stability studies, processing (drying, milling, mixing. granulation etc.) & storage. Mannitoloccurs in four forms, all melting at the same temp. & they are non-hygroscopic, only form B shows a small endotherm exotherm process.[15]
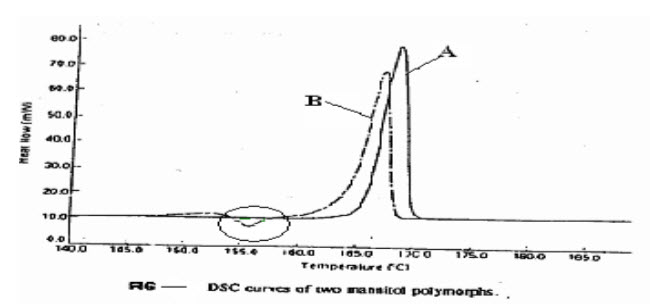
Figure 5: DSC curve for mannitol
The polymorphic behavior of organic substances is driven by thermodynamic and kinetic factors. Therefore several solid phases may coexist. Polymorphic behavior of Donepezil Hydrochloride where three polymorphs were identified (II, III and V) by DSC curves and thermodynamically stable form III was determined (the highest: melting point, enthalpy and true density).
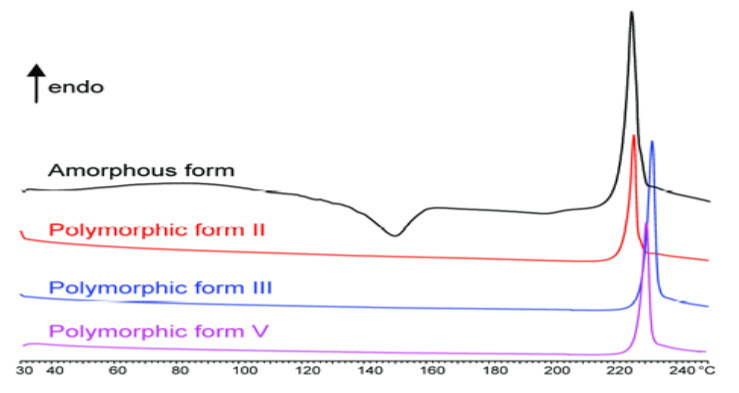
Figure 6: Polymorphic forms of donepezil HCl
In the case of Tripalmitin, the first heating run shows the sample as received is in the stable form. On cooling, a metastable modification crystallizes out, whose melting point lies lower than that of the thermodynamically stable form.
5. Annealing
Isotactic polystyrene (iso-PSt) forms a glassy state when it is quenched from the molten state to 200 K. The DSC heating curve of quenched iso-PSt reveals a glass transition, cold crystallization and melting. By annealing at various temperatures a sub-melting peak can be observed.[10]
6. Prediction of stability of drug
Stability testis one of the tests which req. the longest time in drug development. For a quick release of new drugs to the market, it is difficult to estimate stability speedy from exact information in preliminary stability tests. Thermal analysis is an ideal technique to solve this problem. DSC was used to study the thermal stability of antibodies that were being engineered for improved potency. In this study, we demonstrated a correlation between decreased thermal stability based on DSC data, and greater aggregation formation during accelerated stability studies (at 60°C). The SEC-HPLC stability data agree quite well with the stability predicted by DSC, suggesting that the thermal stability data obtained from DSC correlates with protein stability. Information derived from DSC can indicate potential long-term stability issues, thus making the technique a useful tool in the screening and selection of engineered proteins. Vitamin A is very sensitive to chemical degradation caused by oxygen, light, heat, and other stress factors. If light and oxygen are excluded, the dominant degradation reaction for vitamin A derivatives is heat-induced formation of kitols, that is, dimers or higher oligomers. The vitamin A stability of a given sample can easily be predicted from the initial heat flow in a simple microcalorimetry experiment. Compared to conventional stability tests, this offers savings of money and time.The utility of microcalorimetry as a rapid screening tool for assessing the solution stability of high molecular weight pharmaceutical proteins was evaluated by using model recombinant antibodies, Protein I and Protein II. Changes in the transition midpoint, Tm, were monitored as a function of pH and/or in the presence of excipients, and results were compared with traditional accelerated stability data from samples that were analyzed by size exclusion chromatography (SEC). The data from microcalorimetry were well correlated with those from SEC for predicting both optimal solution pH as well as excipient effects on solution stability.[1-3, 10]
Advantages of thermal analysis in prediction of stability
- This method is very speedy
- It only takes two weeks to predict stability of a drug substance in detail.
- The operations are simple
- The method required very small quantity of drug substance & total amount of sample being necessary to predict stability is very little so method is used even at an early developmental stage when production scale is small
- Furthermore the accuracy & the precision of prediction using the method are equivalent to or better than those of preliminary tests
- This method is very widely applicable to
# Pharmaceutical intermediate
# Pharmaceutical excipient
# Agricultural chemical
# Pesticides
# Pharmaceutical raw material.
7. Degree of crystallinity
It can be determined by IR, X-ray diffraction and density measurement bus DSC is one of the most easiest.as it is exothermic event just take ratio of enthalpy of fusion of sample and crystal so as to get fraction of crystallinity. Isothermal microcalorimetry gas flow vapour sorption experiments can be used to assess the surface energetics of crystals, the formation and/or loss of hydrates as a function of humidity and the onset of deliquescence, and to identify the presence of amorphous content in powders.[1, 10]This directly or indirectly gives idea about the stability of the crystal substance. Partial crystallinity is also a type of polymorphism. The degree of crystallinity was determined by solution calorimetry. Principle of solution calorimetry. Basis on the fact that for many solids the amorphous form is higher in energy then the crystalline form. The heat of solution of the amorphous form is expected to be more exothermic then the crystalline form. The percentage of crystallinity (pc) may be determined in a partially crystalline sample according to the following equation.
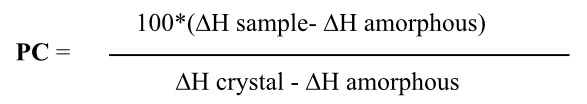
Where, sample is partially crystalline material; amorphous is 100% amorphous standard & crystalline is 100% crystal standard.Microcalorimetry has proved to be an effective analytical technique for characterizing micronized compounds. It can be used to detect the presence of metastable regions not detectable by X-ray diffraction. Furthermore, the kinetic of “recrystallization” of these regions can be studied, making a prediction of physical stabilitypossible.[16]
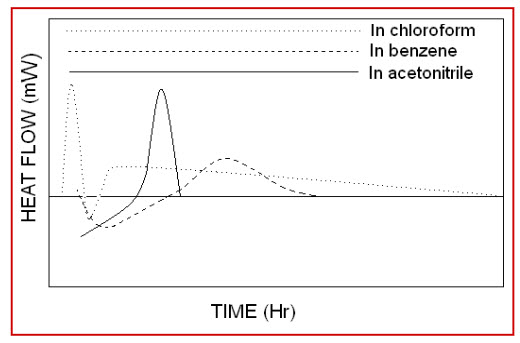
Figure 7: Microcalorimetric spectra of erythromycin in different solvents
Thus the application of Microcalorimetry in the pharmaceutical development has a great potential.The amorphous regions can alter the physical properties of the material, which may have an impact on further pharmaceutical processes or the quality of the material. Microcalorimetry is a very sensitive way to measure amorphous contents well below 1%. The heat of recrystallization of the amorphous parts can be measured and by a suitable calibration the amorphous content can be determined. Solution calorimetry is able to measure directly the heat change caused by the dissolution of a crystalline or partially crystalline powders. The observed heat of solution is a function of the variability in crystallinity displayed. The quantification of amorphous contents, however, requires the availability of pure amorphous and pure crystalline standards.[14]
8. Compatibility study of drug with excipient.
The early detection of Drug excipient Incompatibility is vital in pharmaceutical industry to avoid costly material wastage & time delays. DSC & TGA with the support of x-ray diffraction & infrared spectroscopy are used as screening technique for the compatibility testing of drug with excipient. An isothermal microcalorimeter can be utilized to characterize a model solid-state interaction. The degradation of the HIV protease inhibitor, DMP 450, in a binary mixture with hydrous lactose was followed in the presence of 5% additional water. Heat produced in the microcalorimeter sample vessel from either chemical or physical change (Browning reaction) is channeled through extremely sensitive thermopile blankets and is measured as it flows into infinite heat sinks. DSC of sparfloxacin with PVP shows absence of melting endotherm & exotherm is broad which indicate incompatibility.[2, 3, 17, 18]
9. Phase Diagram
A phase diagram is a graphical representation of the relationship between a given set of experimental parameters and the phase changes occurring in a material. Sample volume, transition temperature and enthalpy, pressure and composition of the material are commonly used parameters in phase diagrams.In the presence of a small amount of water a glass transition, cold crystallization, melting and a liquid crystal transition are observed in case of xanthan gum.[10]
10. Study of complexes & inclusion compounds
The Disappearance of the DSC peak of the drug is the proof of Complexation in solid state.The complexation of bifonazole, an antimycotic hydrophobic imidazole derivative, with β-cyclodextrin (β-CD) was investigated in solid phase, using the following complementary techniques: differential scanning calorimetry (DSC), thermogravimetric analysis (TG) and X-ray powder diffractometry.[19]The thermally stimulated dissociation of the inclusion complex of 1-methylcyclopropene (1-MCP) with alpha-cyclodextrin (alpha-CD) in solid state was studied by means of thermogravimetry (TG) and differential scanning calorimetry (DSC). The mass loss of 1-MCP/alpha-CD inclusion complex occurs in four separated phases with the thermal dissociation of the inclusion complex and release of 1-MCP taking place in the second phase between 90 and 230 degrees C. The kinetic parameters of the dissociation reaction (the apparent activation energy of dissociation, E(D), the reaction order of thermal dissociation, n, and the pre-exponential factor, k0) were evaluated. The dissociation reaction was satisfactorily described by the unimolecular decay law, where the reaction order, n = 1. The effect of the molar ratio of 1-MCP to alpha-CD (inclusion ratio) in the inclusion complex on the temperature dependence of the dissociation reaction was also studied.[20]
11. Study of Thermal behavior Sugar ester
Sugar esters have wide range of HLB value so they can be used as surfactant or penetration enhancer. Thermal analysis can be used to measure thermal properties of Sugar ester & to differentiate sugar ester with HLB. Thermal properties are measured with modulated DSC & combined with hot stage microscopy to visualized changes in sample during heating.Hydrophilic SEs were only softened, while lipophilic SEs were melted by heating. After melting and solidification, SEs have partially amorphous layered structures which slowly crystallize in time. Time-dependent solid-state changes (crystalline and amorphous phases) were observed, and analysed by means of differential scanning calorimetry and X-ray powder diffraction.[21]
12. Research biology
The starting point of any research is the biological target from which chemistry has to flow.The drugs must interact with the biological target to have activity. The qualitative and quantitative measurement of these interactions gives very useful information. Aqueous lipid dispersions have been used as models for biological membranes. DSC is used for characterization of the interaction of model H+ /K+ ATPase inhibitors with a dimmyristoylphosphatidyl – choline (DMPC). The following figure illustrates the effect different inhibitors on the transition onset temperature and the shape of the endotherm of DMPC (Ried et al., Biochimica et Physica Acta-1).
13. Minor applications
- DSC is a valuable tool in choice of suppository base
- In study of polymer composition, miscibility & individual characterization
- Study of tablet coating
- Determinations of melting point
- Checking technological quality grade of disintegrate
- Study of solid drug dispersion
- Determination of drying temperature for different excipients
Conclusion:
There are many innovations are seen in the field of thermal analysis since last decades. Thermal analysis is vital in case of preformulation study. It is utilized for drug identifications and in compatibility studies. Some recent advancements are described in this article with some of its pharmaceutical features in terms of preformulation study.
References
[1] Skoog DA, Hollar J, Nieman H, Principle and Instrumental analysis, Pearson education, 2008.
[2] Murphy DK, Shelley R, Thermal Analysis and Calorimetric Methods for the Characterization of New Crystal Forms, in: Brittain HG (Ed.) Preformulation in Solid Dosage Form Development, Marcel Dekker, 2008.
[3] Gabbott P, Principles and Applications of Thermal Analysis, Blackwell Publishing Ltd., 2008.
[4] Ramos-Sánchez MC, Rey FJ, Rodríguez ML, Martín-Gil FJ, Martín-Gil J, DTG and DTA studies on typical sugars, Themochim Acta, 134 (1988) 55-60.
[5] Hsieh YP, A novel multielemental scanning thermal analysis (MESTA) method for the identification and characterization of solid substances., J AOAC Int., 90 (2007) 54-59.
[6] Craig DQ, Kett VL, Andrews CS, Royall PG, Pharmaceutical applications of micro-thermal analysis, J Pharm Sci. , 91 (2002) 1201-1213.
[7] Skotnicki M, Gawe? A, Cebe P, Pyda M, Thermal behavior and phase identification of Valsartan by standard and temperature-modulated differential scanning calorimetry., Drug Dev Ind Pharm., [Epub ahead of print] (2012).
[8] Ryou H, Pashley DH, Tay FR, Arola D, A characterization of the mechanical behavior of resin-infiltrated dentin using nanoscopic Dynamic Mechanical Analysis., Dent Mater., 29 (2013) 719-728. .
[9] Ford JL, Mann TE, Fast-Scan DSC and its role in pharmaceutical physical form characterisation and selection, Adv Drug Deliv Rev. , 64 (2012) 422-430.
[10] Quinn FX, Thermal Analysis, 2nd ed., Wiley blackwell science, England, 2009.
[11] Birge NO, Nagel SR, Physical Review Letters, 54 (1985) 2674.
[12] Haines PJ, Principles of Thermal Analysis and Calorimetry, RSC paperbacks, 2002.
[13] H¨ohne, Hemminger, Flammersheim, Calorimetry and Thermal Analysis of Polymers, in: Mathot M (Ed.) Differential Scanning Calorimetry, Springer, 2003.
[14] Beezer AE, O’Neill MA, Urakami K, Conner JA, Tetteh J, Pharmaceutical micro-calorimetry: recent advances in the study of solid state materials., Thermochim. Acta, 420 (2004) 19–22.
[15] Yu L, Inferring thermodynamic stability relationship of polymorphs from melting data., J. Pharm. Sci. , 84 (1995) 966–974.
[16] Wunderlich B, Thermal Characterization of Polymeric Materials, 2nd ed., Academic Press, California, 1997.
[17] Clas SD, Dalton CR, Hancock BC, Differential Scanning calorimetry: applications in drug development., Pharm.Sci. Technol. Today, 2 (1999) 311–320.
[18] Varma M, Wunderlich BJ, Nonisothermal heat capacities and chemical reactions using a modulated differential scanning calorimeter., Therm. Anal., 46 (1996) 879–892.
[19] Morin N, Chilouet A, Millet J, Rouland JC, Bifonazole-β-cyclodextrin Inclusion Complexes. Thermal analysis and X-ray powder diffraction study, Journal of Thermal Analysis and Calorimetry, 62 (2000) 187-201.
[20] Neoh TL, Yamauchi K, Yoshii H, Furuta T, Kinetic study of thermally stimulated dissociation of inclusion complex of 1-methylcyclopropene with alpha-cyclodextrin by thermal analysis., J Phys Chem B, 112 (2008) 15914-15920. .
[21] Szuts A, Pallagi E, Regdon G Jr, Aigner Z, Szabó-Révész P, Study of thermal behaviour of sugar esters., Int J Pharm., 336 ( 2007) 199-207.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









