 About Author:
About Author:
Dhananjay S Jadhav*
M.Tech (Pharmaceutical Technology), Division of Pharmaceutical Technology,
Institute of Chemical Technology, North Maharashtra University, Jalgaon -425001.
Maharashtra, India
* dhananjaysjadhav@hotmail.com
Abstract
Recently, surfactants have become one of the most important chemical products. They have become a subject of research, and their production and their use are on the increase. This wide range of properties, uses for surfactants in pharmaceutical products and systems is to try and introduce and explain in the subject of this article. Wetting of Solids, Solubilization, Emulsification, Dispersion of solid in solution, Micellization & Detergency all these are properties of surfactant. Surfactants are classified according to their polar head group, the charged head referred as Ionic surfactants and uncharged surfactants are generally referred to as nonionic surfactant. Because of their unique functional properties, surfactants find a wide range of uses in pharmaceutical preparations. These include, depending on the type of product, improving the solubility or stability of a drug in a liquid preparation, stabilizing and modifying the texture of a semisolid preparation, or altering the flow properties of a granulate, thus aiding in the processing of the final tablet dosage form. In addition to their use as excipients to improve the physical and chemical characteristics of the formulation, surfactants may be included to improve the efficacy or bioperformance of the product. The properties of surfactants are such that they can alter the thermodynamic activity, solubility, diffusion, disintegration, and dissolution rate of a drug. Each of these parameters influences the rate and extent of drug absorption. Furthermore, surfactants can exert direct effects on biological membranes thus altering drug transport across the membrane. The overall effect of inclusion of a surfactant in a pharmaceutical formulation is complex and may be beyond those initially intended. Surfactants may reduce the effectiveness of antimicrobials or preservatives included in a formulation.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1714
INTRODUCTION
Surfactants are widely used in molecular biology in formulating new and in modifying existing medical preparations, in the production of proprietary medicines, in the production of chemical preparations aimed for the domestic use, etc. All this is understandable since the properties of surfactants are to some degree responsible for the existence and the performance of cellular membranes, emulsification, solubilization, and of transport of compounds that are otherwise insoluble in living tissues. Surfactants also function as film formation promoters, as wetting agents, detergents, bactericidal agents, etc.
The number of specialists interested in surfactants is indeed very large and the literature devoted to surfactants is correspondingly wide. However, an adequately comprehensive and contemporary monograph on surfactants is currently not available. This wide range of properties, uses for surfactants in pharmaceutical products and systems is to try and introduce and explain in the subject of this article.[1]
PHYSICOCHEMICAL BACKGROUND
* Surface And Interfacial Tension [2]
Atoms and molecules at surfaces and interfaces possess energies significantly different from those of the same species in the bulk phase.
Generally when two / more phases exist together, the boundary between them is termed an interface. The term ‘‘surface’’ is usually reserved for the region between a condensed phase (liquid or solid) and a gas phase or vacuum, while the term ‘‘interface’’ is normally applied to the region between two condensed phases i.e. solid-solid, solid-liquid and liquid-liquid (immiscible), but gas-gas dose not have surface or interface.
The following are the surface tensions for some liquid substances
|
SUBSTANCE |
SURFACE TENSION |
|
Water |
73 dynes/cm |
|
Mercury |
480 dynes/cm |
|
Benzene |
28 dynes/cm |
|
Ethanol |
22 dynes/cm |
Table No.1: Surface Tensions for Some Liquid Substances
Interfacial tension is the force per unit length existing at the interface between two immiscible liquid phases and like surface tension has the units of dyne / cm.[3]
* Surface And Interfacial Free Energy
The work required to increase the surface area by unit area is termed the surface free energy. At the interface between two condensed phases, the dissimilar molecules in the adjacent layers facing each other have potential energies greater than those of similar molecules in the respective bulk phases. This is due to the fact that cohesive forces between like molecules tend to be stronger than adhesive forces between dissimilar molecules. Thus the interfacial tension is the force per unit length existing at the interface between two immiscible or partially miscible condensed phases and the interfacial free energy is the work required to increase the interface by unit area.[4]
* Surfactants
Surfactants are termed as surface-active agents also wetting agents, emulsifying agents or suspending agents depending on its properties and use.
Surface-active agents are substances which, at low concentrations, adsorb onto the surfaces or interfaces of a system and alter the surface or interfacial free energy and the surface or interfacial tension.[5, 6] Surfactants are monomers, it has a characteristic structure possessingboth hydrophobic groups / non-polar regions (their "tails") usually contain a C12–C18 hydrocarbon chainand hydrophilic groups / Polar Regions(their "heads"). Therefore, they are soluble in both organic solvents and water, so they called amphiphilic.[7]

Fig.no.1. Surfactant (Monomer) Head - Polar, Hydrophilic Tail - Non polar, Hydrophobic
• Adsorption Phenomena of Surfactant[2]
Adsorption may be defined as the process of enrichment of one or more substances at a surface or as the taking up of one substance at the surface of another. It can occur at any type of interface. However, in the context of pharmaceutical systems the interfaces where surfactant adsorption is important are the liquid–liquid, gas–liquid, gas–solid, and liquid–solid interfaces.
• Adsorption At Liquid-Liquid Interfaces
The surface tension of any liquid depends on the strength of intermolecular interactions within that liquid. E.g. Water having strong intermolecular interactions (hydrogen bonds), has a surface tension of 73 mN m-1; on the other hand, heptane with much weaker intermolecular interactions (van der Waal’s forces), has a surface tension of only 20 mN m-1.
Considering a system of two immiscible phases (e.g. heptane and water), a surface active molecule that is adsorbed at the interface between the two liquids will tend to orient itself with its hydrophilic end toward the more polar liquid (water), and its hydrophobic end toward the less polar liquid (heptane). Thus the surfactant molecules replace water and/or heptane molecules of the original interface. The interaction across the interface is then between the hydrophilic group of the surfactant and the water molecules on one side of the interface, and between the hydrophobic group of surfactant and heptane on the other side of the interface. These interactions are much stronger than the original interactions between the unlike molecules of heptane and water; therefore the interfacial tension is significantly reduced by the adsorption of surfactant at the interface i.e., the inward pull for each phase at the interface is reduced.
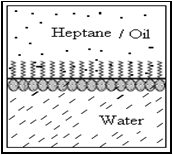
Fig.No.2 Alignment Of Surfactant At A Liquid- Liquid Interface.
• Adsorption At Liquid-Gas Interfaces
Air consists of molecules that are mainly non-polar and water molecules are polar with high surface tension. Surface tension reduction by surfactants at the air– aqueous interface occurs due to adsorption of surfactants at the interface.
[adsense:468x15:2204050025]
When a surfactant is dissolved in water, the presence of the hydrophobic portion in solution disrupts the balance of the intermolecular forces in the bulk liquid. This leads to a rise in the free energy of the system. This means that it is easier (requires less work) to promote a surfactant molecule than a water molecule to the surface. The surfactant molecules therefore congregate at the surface with their hydrophobic tails aligned in the air and hydrophilic head with water, due to strong intermolecular interactions (hydrogen bonds) of surfactant with water. i.e. The presence of the surfactant molecules reduces the net inward pull toward the bulk liquid, and therefore reduces the surface tension.

Fig.No.3. Alignment of Surfactant At A Liquid- Gas Surface.
In extremely dilute solutions, the drop in surface tension is proportional to the surfactant concentration. However, at a certain concentration, the surface tension drop stops. Surface is saturated, as the concentration increases but the surface tension remains almost constant. After that the point at which this behavior change is observed and surfactants goes in solution to form aggregation of surfactant i.e. micelle. This is called the Critical Micelle Concentration.[8]

Fig.no.4. CMC Data for Sodium Dodecyl Sulfate.
Physically, what is happening here is the following; surfactant molecules congregating at the surface gradually cover more and more of that surface as their concentration in the solution increases - surface tension drops proportionally. At the point where the surface becomes saturated, micellization occurs in the bulk liquid. The number of surfactant molecules at the surface reaches a maximum, i.e. above CMC - the surface tension remains constant.
• Adsorption At Solid–Liquid Interfaces
Adsorption of surfactant from an aqueous solution onto a solid surface may involve specific chemical interaction between the surfactant (adsorbate) and the surface (adsorbent).
Common interactions that can occur include:
- An ion-exchange process
- An ion-pairing interaction
- Acid–base interaction via either hydrogen bonding between substrate and adsorbate or Lewis acid–Lewis base reaction
- Adsorption by dispersion forces, i.e., London– van der Waals dispersion forces acting between adsorbate and adsorbent
- Hydrophobic bonding.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PROPERTIES OF SURFACTANT[3,13]
• Wetting of Solids
• Solubilization
• Emulsification
• Dispersion of solid in solution
• Micellization
• Detergency
• Micellization
Definition- A micelle is an aggregate of surfactant molecules dispersed in a liquid colloid. The process of forming micelle is known as micellization. Micelle formations in polar and non-polar solvent depend on the concentration of the surfactant in the particular solvent. If add surfactant (monomers) in solvent, at low concentration some monomers dispersed in solvent or aggregate at the surface or interface until all surface or interface saturated by surfactant. Further addition of surfactant increase concentration of monomers and it goes in solvent and start to form micelles. This concentration is called CMC. [Critical micelle concentration]
CMC- The concentration of monomer at which the micelles are start to form in solvent at particular temperature. Micelles form only when the concentration of surfactant is greater than the critical micelle concentration (CMC).[10,11]
Micelles are divided into Monomeric micelle, Reverse micelle, Polymeric micelle depending upon the type of the solvent (polar and non-polar) used for the formation of micelle.
• Monomeric Micelle
Individual surfactant molecules that are in the system but are not part of a micelle are called "monomers." In micelle, the hydrophobic tails of several surfactant molecules assemble into an oil-like core the most stable form of which has no contact with water. By contrast, surfactant monomers are surrounded by water molecules that create a "cage" of molecules connected by hydrogen bonds. This water cage is similar to ice-like crystal structure.
Micelles are dynamic species; there is a constant rapid interchange of surfactant molecules between the micelle and the bulk solution. Micelles cannot, therefore, be regarded as rigid structures with a defined shape, although an average micellar shape may be considered and Micelles are labile entities formed by the non-covalent aggregation of individual surfactant monomers. Therefore, they can be spherical, cylindrical, or planar (discs or bilayers). Micelle shape and size can be controlled by changing the surfactant chemical structure as well as by varying solution conditions such as temperature, overall surfactant concentration, surfactant composition (in the case of mixed surfactant systems), ionic strength and pH. In particular, depending on the surfactant type and on the solution conditions, spherical micelles can grow one-dimensionally into cylindrical micelles or two-dimensionally into bilayers or discoidal micelles. Spherical micelles exist at conc. relatively close to the CMC. At higher conc. lamillar micelles have an increasing tendency to form and exist in equilibrium with spherical micelle.
Micelle growth is controlled primarily by the surfactant heads, since both one dimensional and two dimensional growth require bringing the surfactant heads closer to each other in order to reduce the available area per surfactant molecule at the micelle surface, and hence the curvature of the micelle surface.
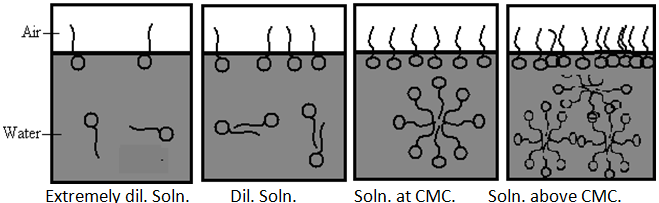
Fig.No.5. The Formation Of Micelle Form Monomer (Surfactant)
In polar solvent, the hydrophilic "heads" of surfactant molecules are always in contact with the sequestering solvent and the hydrophobic single tail regions in the micelle centre called normal micelle (oil-in-water micelle).This phase is caused by the insufficient packing issues of single tailed lipids in a bilayer. The difficulty filling all the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group leads to the formation of the micelle.
One of the most important applications of micellization in the context of pharmaceuticals is their ability to solubilize drugs of poor aqueous solubility.[8,11]

Fig.No.6. Micelle In Polar Solvent [7]
• Reverse micelle
In a non-polar solvent, the lipophilic "tails" of surfactant molecules have less contact with water or the exposure of the hydrophilic head groups to the surrounding solvent that is energetically unfavorable. Therefore, the head groups are pulls at the centre with the tails extending out called as Inverse\ Reversemicelle (water-in-oil micelle). Dipole–dipole interactions hold the hydrophilic heads of the surfactant molecules together in the core, and in certain cases hydrogen bonding between head groups can also occur. Reverse micelle formed by the aggregation of the 3 to 20 monomer by oil soluble surfactant. e.g. Scheme of an inverse micelle formed by phospholipids in an organic solvent.

Fig.No.7. Reverse Micelle (In Non Polar Solvent)[7]
In the same way that normal micellar systems can be used for solubilizing hydrophobic substances in an aqueous solution, reversed micellar systems may be used for solubilizing water-soluble drugs in an oil-continuous system.[13,14,15]
• Polymeric micelle
In drug delivery, special attention has been given to the polymeric micelles. Polymeric micelles are composed of block or graft copolymers. Polymeric micelles are formed from copolymers consisting of both hydrophilic and hydrophobic monomer units, such as PEO and PPO (polyethylene oxide and polypropylene oxide), respectively. These amphiphilic block co-polymers with the length of the hydrophilic block exceeding the length of the hydrophobic block can form spherical micelles in aqueous solution. The micellar core consists of the hydrophobic blocks and the shell region consists of the hydrophilic blocks. The PEO coating has been shown to prevent subsequent recognition by the macrophages of the reticuloendothelial system (RES), allowing the micelles to circulate longer and deliver drugs more effectively to the desired sites. Block copolymers are generally linear polymers that are composed of a sequence of at least two polymer segments that differ in physico-chemical properties, e.g. charge and/or polarity.
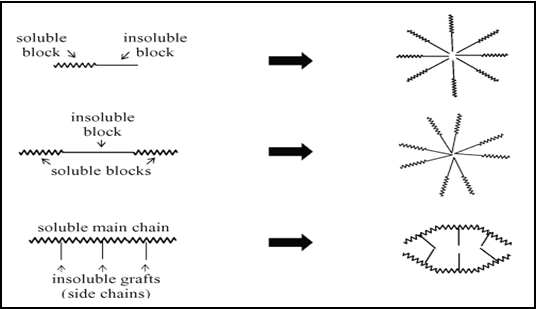
Fig.No.8.Represents A Schematic Representation Of The Mechanism Of Block Polymeric Micelles Formation.[16]
Formations of polymeric micelles from different types of amphiphilic block co-polymers. In graft copolymers, side chain segments are grafted to a main polymer chain. Fully hydrophilic block or graft copolymers in which one of the segments carry a charge may form stable complexes in water together with oppositely charged (macro) molecules, resulting in, for instance, so-called polyion complex micelles or polyelectrolyte micelles.[17] On the other hand, so-called amphiphilic block copolymers are capable to self-assemble, when placed in a solvent that is selective for one of the polymeric segments, to form micelles, vesicles or gels. In an aqueous environment, such self-assembled structures are interesting for encapsulation and (controlled) release of a variety of drugs.[18]
Drug loading of amphiphilic block copolymer micelles is generally accomplished by
a) Dissolving the polymer and the hydrophobic drug in an organic solvent and subsequent dialyzing against water or diluting in water and evaporating the solvent.
b) Another method that is reported occasionally is the ‘spontaneous’ hydration of a polymer/drug mixed film.
⇒ Advantages of Polymeric Micelle Over Monomeric Micelle
Polymeric micelles, which are either formed from block copolymers by ionic or by hydrophobic interactions have several advantageous features that make them interesting as drug delivery systems. Some of them mention below
• Polymeric carriers might lead to precipitation in water, since the drug-polymer interaction can result in conversion of functional water-soluble groups of the drug into more hydrophobic groups.
• Polymeric micelles refer to the easy for sterilization via filtration and safety for administration.
• Some polymeric micelles seem to present better solubilization capacity when compared to surfactant micelles due to the higher number of micelles and/or larger cores of the formers.
• The slow dissociation of kinetically stable polymeric micelles allows them to retain their integrity and perhaps drug content in blood circulation above or even below the CMC for some time, creating an opportunity to reach the target site before decaying into monomers.
• Well defined core shell architecture. For example, the core of amphiphilic block copolymer micelles is capable to accommodate (solubilize) poorly water soluble drugs that are otherwise difficult to administer to the body. The hydrophilic shell provides colloidal stability to the whole assembly.
• Small size (< 150 nm). Since the smallest blood vessels in the body (the capillaries) have a diameter of approx. 200 nm, polymeric micelles are able to freely circulate in the blood stream after injection of the drug solubilisate.
• High physical stability. Because the critical micelle concentration (CMC) of a block copolymer can be several orders of magnitude lower than that of a classical surfactant, the micelles are highly resistant against dilution that unavoidable when administered to the patient (e.g. by injection).[17]
* Wetting of Solids
A drop of liquid when placed on a flat, homogeneous solid surface comes to equilibrium, assuming a shape which minimizes the total free energy of the system. The angle between the liquid and the solid is called the contact angle (θ), the angle being measured through the liquid. The contact angle may be calculated if the surface and interfacial tensions are known from Young’s equation given in Eq. (1) or (2).
γSA = γSL + γLA cos θ ______________________________(1)
Or
Cosθ = γSA – γSL / γLA ______________________________(2)
Where γLA is the surface tension of the liquid, γSL is the interfacial tension existing between the solid and liquid phases, and γSAis the surface tension (or surface free energy) of the solid. If θ< 900, wetting of the solid is said to take place. If θ> 900, wetting does not take place. The term ‘‘wetting’’ refers to the displacement from a surface of one fluid by another. It is most commonly applied to the displacement of air from a liquid or solid surface by water or an aqueous solution. The term ‘‘wetting agent’’ is applied to any substance that increases the ability of water or an aqueous solution to displace air from a liquid or solid surface. For good wetting, cosθshould be as close as possible to 1; that is, θshould be as close as possible to 0.
From Young’s equation, it can be seen that if γLA or γSL was minimized, cosθ would be maximized, and wetting would be promoted. Contact angles of water on powders of pharmaceutical importance are usually measured by preparing disks of the powder by compression or melting.
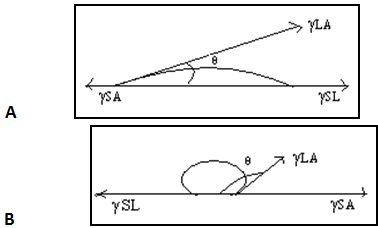
Fig.no.9. Contact angles of liquid on surface.
However, compaction may change the surface, so making the measured result of little relevance. Contact angles on finely divided solids can also be determined by packing the powder into a tube and measuring the penetration of liquids into the packing. Three types of wetting phenomena have been described:
· Adhesional wetting
· Spreading wetting
· Immersional wetting.
The way in which a particular system behaves depends on the interfacial energies between the solid substrate and any contacting liquid, and between the liquid and the second fluid (air). By manipulating these factors, the wetting process can be controlled. This may be achieved by the use of surfactants.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Modification of the wetting process by the use of surfactants, the effect of surfactants on the wetting process is a result of their adsorption at various interfaces with a resulting alteration of interfacial tensions. As has been noted from Young’s equation, the wetting process is promoted if either γLAor γSL or both are reduced with γSA remaining unchanged. Surfactants almost always cause a reduction in γLA, however, the same cannot be said for γSL and the effect on the interfacial tension depends on the nature of the adsorption. Thus the addition of a surface-active agent to the system does not always promote wetting, and spreading may in fact be made more difficult. If adsorption of the surfactant molecules at the solid–liquid interface occurs in such a manner that they are oriented with their polar ends toward the substrate and hydrophobic ends toward the liquid, the wettability of an aqueous solution is reduced. This orientation of surfactants molecules at the surface occurs if they are adsorbing to ionic or polar substrates (ion-exchange or ion-pairing mechanism). However, at higher concentrations of surfactant, the surfactant ions adsorb by hydrophobic interaction with the already adsorbed layer, thus exposing their hydrophilic ends to the solution in such a way that the surface becomes more readily wetted. Thus, the contact angle may first increase and subsequently decrease following the addition of more surfactant to a solution. In contrast, where adsorption occurs onto non-polar surfaces by, for example, van der Waals attraction, the surfactant molecules are oriented with their hydrophilic groups toward the liquid, the hydrophilicity of the substrate is increased, and it becomes more wettable.
The adsorption of surfactants onto solid surfaces is important with respect to their detergent properties, their use as wetting agents in solid pharmaceutical dosage forms, and as stabilizers for suspension formulations. The mode of action of surfactants in each of these systems is discussed further below.[2, 7, 19]
* Solubilization
Solubilization can be defined as ‘‘the preparation of a thermodynamically stable isotropic solution of a substance normally insoluble or very slightly soluble in a given solvent by the introduction of an additional amphiphilic component or components.’’ The amphiphilic components (surfactants) must be introduced at a concentration at or above their critical micelle concentrations. Simple micellar systems (and reverse micellar) as well as liquid crystalline phases and vesicles referred to above are all capable of solubilization. In liquid crystalline phases and vesicles, a ternary system is formed on incorporation of the solubilizate and thus these anisotropic systems are not strictly in accordance with the definition given above.
Solubilization by micelles
The location of a solubilized molecule in a micelle is determined primarily by the chemical structure of the solubilizate. Solubilization can occur at a number of different sites in a micelle:
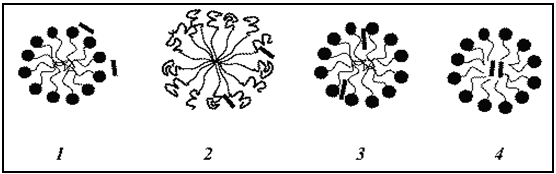
Fig.No.10.
In Aqueous SystemsSolubilization Of Drugs At Diff. Positions Of Micelle.[19]
1. On the surface, at the micelle–solvent interface,
2. At the surface and between the hydrophilic head groups,
3. In the palisades layer, i.e., between the hydrophilic groups and the first few carbon atoms of the hydrophobic groups that comprises the outer regions of the micelle core.
4. More deeply in the palisades layer, and in the micelle inner core.
Examples
1. Polar alcohols are soluble in aqueous solution, so it located in solution / on surface of micelle.
2. Phenol are having polar –OH group and non polar benzene ring. In which –oh gr. Located in hydrophilic environment and benzene ring in hydrophobic environment, so it located at the surface and between the hydrophilic head groups.
3. Semipolar materials, such as fatty acids are usually located in the palisades layer, the depth of penetration depending on the ratio of polar to non-polar structures in the solubilisate molecule.
4. Non-polar additives such as hydrocarbons tend to be intimately associated with the hydrocarbon core of the micelle.[17,18]
In non aqueous system
Reverse micelles formed in non-polar solvent systems containing surfactant, polar additives may be solubilized in the core where a polar interaction of head groups occurs. A preferred location of the solubilisate molecule within the micelle is largely dictated by chemical structure.
However, solubilized systems are dynamic and the location of molecules within the micelle changes rapidly with time. Solubilization in surfactant aqueous systems above the critical micelle concentration offers one pathway for the formulation of poorly soluble drugs. From a quantitative point of view, the solubilization process above the CMC may be considered to involve a simple partition phenomenon between an aqueous and a micellar phase. Thus the relationship between surfactant concentration Csr and drug solubility Cdss is given by Eq. (1).
Cdss = Cdsa + P Cdsa Csr ________________(1)
Where Cdss is the drug solubility in the absence of surface active agent and P is the distribution coefficient of drug between the micelle and bulk phases. A plot of Cdss versus Cs is linear with a slope of P Cdsa, which is the solubilizing capacity of the micelle. The effect of altering the pH of the vehicle, in the case of a partly ionized drug will be to alter the apparent partition coefficient. Thus the effect of increasing the pH of a vehicle containing an acidic drug is to reduce the proportion of drug in the micellar phase. If the surfactant is a weak electrolyte, it may induce a concentration-dependent change in pH thus altering drug partitioning and solubility. In general the solubilizing capacity for surfactants with the same hydrocarbon chain length increases in the order anionic < cationic < non-ionic, the effect being attributed to a corresponding increase in the area per head group, leading to looser micelles with less dense hydrocarbon cores which can accommodate more solute.
The solubilizing capacity for a given surfactant system is a complex function of the physicochemical properties of the two components which, in turn, influence the location or sites where the drug is bound to the micelle. The molar volume of the solubilisate together with its lipophilicity is important factors, the former reducing and the latter increasing solubilization.
Many pharmaceutical products contain a number of solutes potentially capable of being solubilized within the micellar phase. Thus competition can occur between solutes resulting in an altered solubilizing capacity. Furthermore, the addition of a second highly solubilized component to form a mixed micellar system may greatly alter the structure, size and solubilizing capacity of the system, thereby greatly enhancing drug solubility.[20]
Pharmaceutical Examples of solubilisation
- The solubilization of phenolic compounds such as cresol, chlorocresol, chloroxylenol and thymol with soap to form clear solutions for use in disinfection.
- Solubilised solutions of iodine in non-ionic surfactant micelles (iodophors) for use in instrument sterilization.
- Solubilisation of drugs (for example, steroids and waterinsoluble vitamins), and essential oils by non-ionic surfactants (usually polysorbates or polyoxyethylene sorbitan esters of fatty acids).[17]
* Detergency
It is most important property of surface active agents. Surface active agents are referred as detergents. The term Detergency is mostly used in the cleaning / removing of grease, oil and dirt from the solid surface. The principle of detergency is based on the formation of micelle.
The process needs many of the actions specific to surfactant molecules.
1. The surfactant requires good wetting properties to ensure good contact with the solid surface.
2. It also has the ability to remove dirt into the bulk liquid.
This property is achieved by lower the surface tension of the medium in which surfactants is dissolved. By lowering this interfacial tension between two media or interfaces (e.g. air/water, water/stain, stain/fabric) the surfactant plays a key role in the removal and suspension of dirt. The lower surface tension of the water makes it easier to lift dirt and grease off of dirty dishes, clothes and other surfaces, and help to keep them suspended in the dirty water. The water-loving or hydrophilic head remains in the water and it pulls the stains towards the water, away from the fabric. The surfactant molecules surround the stain particles, break them up and force them away from the surface of the fabric. They then suspend the stain particles in the wash water to remove them.If the dirt is oily it may be emulsified or solubilized by the surfactant.[2,7,21,22,25]
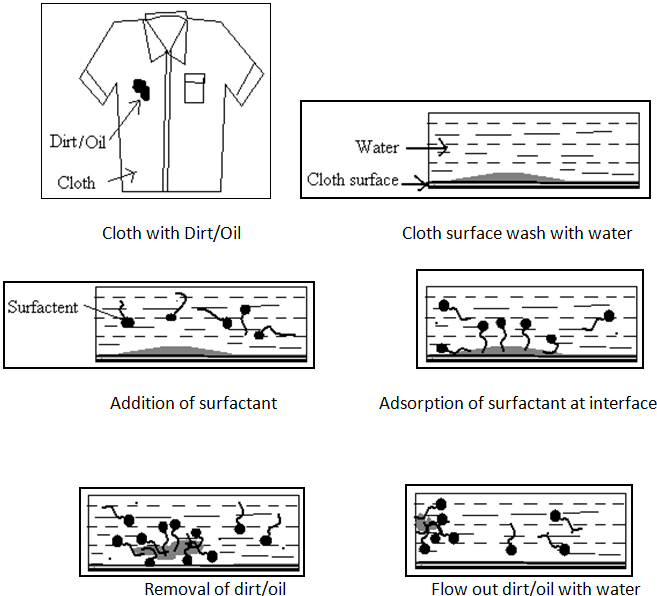
Fig.No.11. Process of Action Of Detergents.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
* Emulsification
This is the property of surfactants to form a stable emulsion of two or more immiscible liquids. This is a little like micellar solubilization, but the resultant solubilizing particles are much bigger.
When oil and water mixed together and agitated, droplets of varying size are produced. Tension exists at the interface because the two immiscible liquid phases tend to have different attractive forces for a molecule at the interface. Molecules of one phase are repelled by other phase due to greater interfacial tension, but for dispersion of liquid necessary of reduction of interfacial tension. This is done by addition of surfactants and having following mechanisms.
• Reduction of interfacial tension – Thermodynamic stabilization.
• Formation of interfacial film – mechanical barrier to coalescence.
• Formation of electrical double layer – electrical barrier to approach of particles.
♦ Interfacial Tension
Even though reduction of interfacial tension lowers the interfacial free energy produced on dispersion. Surfactants are adsorbing on the interface of them, because hydrophilic head have affinity towards water and hydrophobic tail towards oil. This is responsible for reduction of interfacial tension and two immiscible phases are become miscible.
♦ Interfacial Film
The formation of film by the emulsifier is similar to adsorption of surfactants at the interface of an oil and water. If the concentration of emulsifier is high enough, it forms a rigid film between the immiscible phases which act as a mechanical bar to both adhesion and emulsifier of emulsion droplets. In O / W emulsions, the mixture of sodium cetyl sulfate and cholesterol form more stable interfacial film.
♦ Electrical Repulsion / Electrical Double Layer
It has just been described how interfacial films significantly alter the rate of coalescence of droplets by acting as barriers. In addition, the same or similar film can produce repulsive electrical forces between approaching droplets. Such repulsion is due to an electrical double layer, which may arise from electrically charged groups oriented on the surface of emulsified globules.
Consider O / W emulsion stabilized by sodium soap. Not only are the molecules of this surfactant concentrate in the interface, but because of their polar nature they are oriented as well (Fig. no.11). The hydrocarbon tails dissolve in oil droplet, while the ionic heads facilitate the continuous aqueous phase. As a result, the surface of droplet is studded with charged groups, in this case negatively charged carboxylate groups. This produces a surface charge on the droplet; while cations of positive sign are oriented near the surface is known as double layer of charge. The potential produced by the double layer creates a repulsive effect between the oil droplets and avoid coalescence of droplets.[5,21,24]
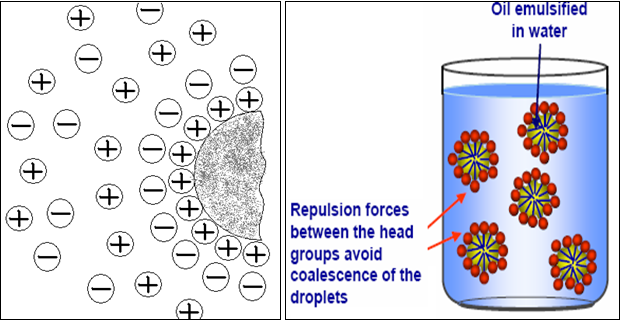
Fig.No.12. Idealized Representation Of The Electrical Double Layer At En Oil Water Interface And Its Effect.
♦ Dispersion of solid in aqueous solution
It is an important property of surfactants that keeps insoluble particles in suspension, so surfactants are referred as suspending agents. They do this by preventing insoluble particles from aggregating with each other. The smaller the particles, the more stable the dispersion formed. This property based on combination of Wetting of solid, Electrical double layer, Nernst and zeta potentials.
Wetting of solid and Electrical double layer are previously seen.
Nernst and zeta potentials
At solid-liquid interface aa’ are surface of solid having positively charge. bb’ are the shear plane having the tightly bounded negative ions between aa’ to bb’. The potential at bb’ are positive charge surface also called true surface. From bb’ to cc’ have excess negative ions and beyond cc’ the ions are uniformly distributed, so it is neutrality is obtained. This electrical distribution at the interface is equivalent to double layer of charge. Curves are shown for three cases characteristic of the ions or molecules in the liquid phase. Note that although E is the same in all three cases, the zeta potentials are positive (z1), zero (z2) and negative (z3).
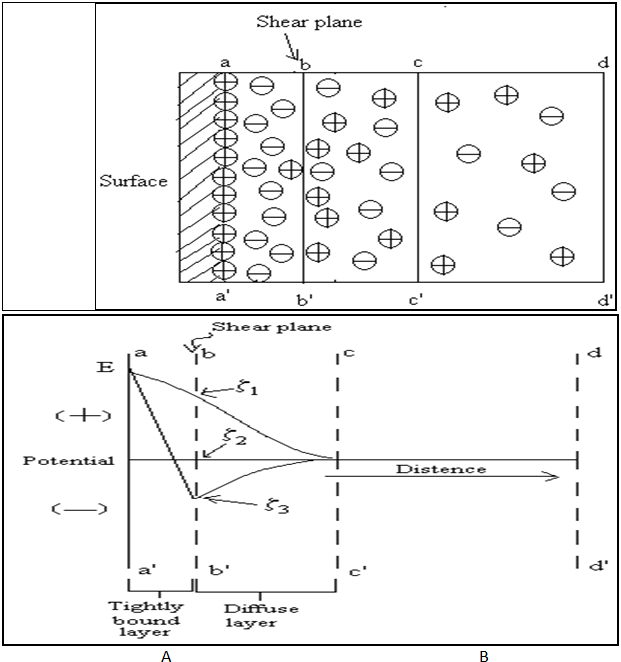
Fig.No.13.A - Electric Double Layer At Interface Of Solid Particle And Liquid.
B - Electro kinetic Potential At Solid-Liquid Boundaries.
The changes in potential with distance from the surface seen in fig.no.10.B. The potential at solid surface aa’, due to the potential-determining ion, is the electrothermodynamic (Nernst) potential (E) and is defined as the difference in potential between the actual surface and electroneutral region of the solution. The potential located at the shear plane bb’ is known as the Electrokinetic or zeta potential (z). The zeta potential defined as the difference in potential between the surface of tightly bound layer (shear plane) and electroneutral region of the solution. As shown in fig. no. 10. B. Zeta potential has practically applied in stabilization of dispersed system in formulation. If the zeta potential is reduced below certain value, the attractive forces exceed the repulsive forces, and particles come together. This phenomenon is known as flocculation.
If surfactant added in the dispersed system (suspension), the zeta potential value is increased, this is resulted in to increase the repulsive forces by adsorbing at surface.[5,21,25]

Table. No. 2 Dispersed System Due To Zeta Potential Value And Repulsive Forces By Adsorbing At Surface
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Classification[2,3,6,7,19,20]
A surfactant can be classified by the presence of formally charged groups in its head. A surfactant carries a net charge in the head called an ionic surfactant. A non-ionic surfactant has no charge groups in its head. If the charge is negative, the surfactant is more specifically called anionic; if the charge is positive, it is called cationic.
Classification Based On Chemical Nature
Surfactants are classified according to their polar headgroup, the charged head referred as Ionic surfactants and uncharged surfactants are generally referred to as nonionic surfactant.
⇒ Ionic
a. Anionic
1. Fatty acid salts (‘‘soaps’’)
2. Sulfates: Sodium dodecyl sulfate (SDS), ammonium lauryl sulfate, and other alkyl sulfate salts, Sodium laureth sulfate, also known as sodium lauryl ether sulfate (SLES)
3. Ether sulfates: Alkyl ether sulfates
4.Phosphate esters
5. Sulphonates: Alkyl benzene sulphonates
b. Cationic
1. Amine salt
• Alkyl amine salt
• Alkyl diamine salt
2. Ammonium Salt
• Alkyl trimethyl ammonium salt
3. Benzalkonium chloride (BAC)
c. Zwitterionic (amphoteric)
1. Quarternary amine group and a carboxyl group contaning surfactent
• Alkyl betaine
• Alkyl imidazoline
2. Quarternary amine group and a sulfonic group contaning surfactent
• Alkyl sulphobetaine
3. Phospholipids surfactant
• Phosphatidyl serine
• Phosphatidyl choline
• Phosphatidyl ethanolamine
4.Carbohydrate-based surfactant
• Alkyl Polyglucoside
• Alkyl Glucamide
⇒ Nonionic
• Alkyl ethoxylate
• Nonylphenol ethoxylate
• Amine ethoxylate
• Alkyl poly(ethylene oxide)
Alkylphenol poly(ethylene oxide)
• Copolymers of poly(ethylene oxide) and poly(propylene oxide) (commercially called Poloxamers or Poloxamines)
Fatty alcohols
- Cetyl alcohol
- Oleyl alcohol
Cocamide MEA, cocamide DEA
Polysorbates: Tween 20, Tween 80
Dodecyl dimethylamine oxide
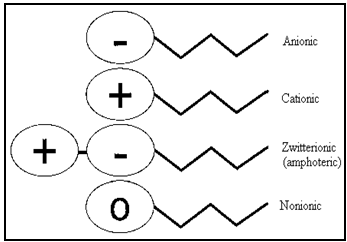
Fig.No.14. Types of Surfactant
Applications
SURFACTANT USED IN PHARMACEUTICAL PREPARATIONS
Because of their unique functional properties, surfactants find a wide range of uses in pharmaceutical preparations. These include, depending on the type of product, improving the solubility or stability of a drug in a liquid preparation, stabilizing and modifying the texture of a semisolid preparation, or altering the flow properties of a granulate, thus aiding in the processing of the final tablet dosage form. In addition to their use as excipients to improve the physical and chemical characteristics of the formulation, surfactants may be included to improve the efficacy or bioperformance of the product. The properties of surfactants are such that they can alter the thermodynamic activity, solubility, diffusion, disintegration, and dissolution rate of a drug. Each of these parameters influences the rate and extent of drug absorption. Furthermore, surfactants can exert direct effects on biological membranes thus altering drug transport across the membrane. The overall effect of inclusion of a surfactant in a pharmaceutical formulation is complex and may be beyond those initially intended. Surfactants may reduce the effectiveness of antimicrobials or preservatives included in a formulation. They also have the capacity to damage biological membranes.[2, 5, 24]
* LIQUID SYSTEMS
Formulation of Solution
Surfactants used in Formulation of Solution as solubilizing agent, which increase Drug solubility. It includes Sorbitan mono oleate and PEG. It used in rang 0.05-0.5% to avoid toxicity.[26]
• Formulation of Suspension (Dispersants)
If the suspension is to be produced by a dispersion technique (as opposed to precipitation techniques), surfactants may be used in the formulation to aid dispersion of the solid particles in the liquid. This is particularly important if the powder is not readily wetted by the liquid vehicle. Surfactants can reduce the interfacial tension between the solid particles and the liquid vehicle. The advancing contact angle is reduced, and wetting of the solid particles promoted. Such a system is said to be deflocculated. The inclusion of a surface-active agent to improve powder wettability can often improve the bioavailability of the formulation. The forces at the surface of a particle affect the degree of flocculation and agglomeration in a suspension. Particles dispersed in a liquid medium may become charged in one of two main ways. Ionic species present in solution may be adsorbed at the surface or, alternatively charges on the surface may arise due to ionization of groups (such as carboxyl groups for example) which may be located at the surface. The surface charge will influence the distribution of ions in the aqueous medium surrounding the solid particles.
The result is the formation of what is known as an ‘‘electric double layer.’’ If the surface charge is positive, immediately adjacent to the surface will be a region of tightly bound solvent molecules and negative counter ions. Thus, the first layer is tightly bound, while the second layer (which still contains an excess of negative ions) is more diffuse.[13] As two particles approach each other in aqueous medium, a weak attractive force exists just beyond the range of the double layer- repulsive forces. This region is responsible for the particle interaction termed ‘‘flocculation.’’
Use as flocculating agent
Flocculated particles are weakly bound, settle rapidly, so do not form a cake and are easily resuspended. For this reason it is frequently desirable to promote flocculation in a suspension. The inclusion of surfactants in the formulation is one way of achieving what is known as ‘‘controlled flocculation.’’ Surfactants can cause dispersed solids to flocculate by a number of different mechanisms. The first is where there is an electrostatic attraction of surfactant ions to oppositely charged sites on the particle surface, resulting in a lowering of the electrical energy barrier to the close approach of two particles to each other. Flocculation may also occur by a bridging mechanism. A long (usually polymeric) surfactant molecule containing functional groups at various sites may adsorb onto sites on the surface of adjacent particles, holding the particles together in a loose arrangement.
Alternatively if the surfactant molecules adsorb in such a manner that the molecule extends into the liquid phase, interaction of the extended portions of surfactant molecules adsorbed to different particles result in bridging of those particles.
Another method of employing surfactants to achieve flocculation is to first treat the particles with an ionic surfactant to disperse them. A readily soluble electrolyte is then added which has the effect of compressing the electrical double layer surrounding each particle, allowing flocculation to occur. Subsequent dilution of this type of system will redisperse it (due to a decrease in electrolyte concentration).[2,6,7,19]
I. Formulation of Emulsions
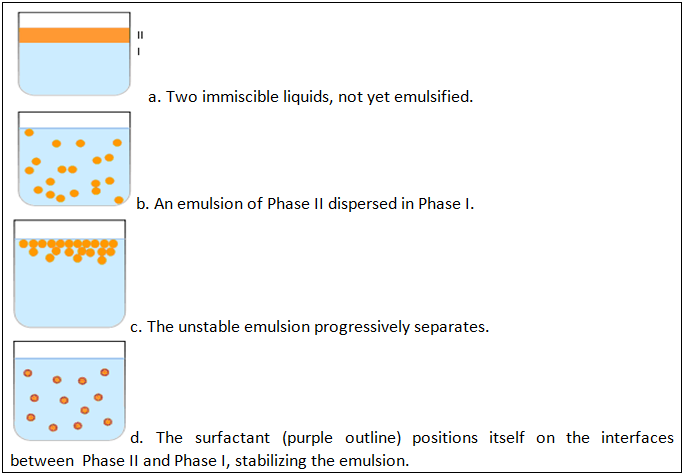
An emulsion is a mixture of two or more immiscible (unblendable) liquids. One liquid (the dispersed phase) is dispersed in the other (the continuous phase). Many emulsions are oil/water emulsions, with dietary fats being one common type of oil encountered in everyday life. Examples of emulsions include butter and margarine, milk and cream. In butter and margarine, fat surrounds droplets of water (a water-in-oil emulsion). In milk and cream, water surrounds droplets of fat (an oil-in-water emulsion).
Emulsion is also a term used in the hydrocarbon industry as untreated well production that consists primarily of crude oil and water. Emulsions tend to have a cloudy appearance, because the many phase interfaces (the boundary between the phases is called the interface) scatter light that passes through the emulsion. Emulsions are unstable and thus do not form spontaneously. Energy input through shaking, stirring, homogenizing, or spray processes are needed to form an emulsion. Over time, emulsions tend to revert to the stable state of the phases comprising the emulsion. Surface active substances (surfactants) can increase the kinetic stability of emulsions greatly so that, once formed, the emulsion does not change significantly over years of storage. Vinaigrette is an example of an unstable emulsion that will quickly separate unless shaken continuously. This phenomenon is called coalescence, and happens when small droplets recombine to form bigger ones. Emulsions can also suffer from creaming, the migration of one of the substances to the top of the emulsion under the influence of buoyancy or centripetal force when a centrifuge is used.
Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion tends to imply that both the dispersed and the continuous phase are liquid.[7]
There are three types of emulsion instability:
- Flocculation, where the particles form clumps;
- Creaming, where the particles concentrate towards the surface (or bottom, depending on the relative density of the two phases) of the mixture while staying separated; and
- Breaking and coalescence where the particles coalesce and form a layer of liquid.
Whether an emulsion turns into a water-in-oil emulsion or an oil-in-water emulsion depends on the volume fraction of both phases and on the type of emulsifier. Generally, the Bancroft rule applies: emulsifiers and emulsifying particles tend to promote dispersion of the phase in which they do not dissolve very well; for example, proteins dissolve better in water than in oil and so tend to form oil-in-water emulsions (that is they promote the dispersion of oil droplets throughout a continuous phase of water).
The basic color of emulsions is white. If the emulsion is dilute, the Tyndall effect will scatter the light and distort the color to blue; if it is concentrated, the color will be distorted towards yellow. This phenomenon is easily observable on comparing skimmed milk (with no or little fat) to cream (high concentration of milk fat). Microemulsions and nanoemulsions tend to appear clear due to the small size of the disperse phase.
Emulsification is the process by which emulsions are prepared. Emulsifiers are the surfactants used for Emulsification.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Use as Emulsifying Agent
An emulsifier (also known as an emulgent) is a substance which stabilizes an emulsion. There is large no. of emulsifying agents which are available to prepare stable emulsion but difficulty to select proper emulsifying agent. For selection of proper agent use of HLB System is must. Sometimes it is necessary to use more than one agent for stability of the preparation.
Examples of food emulsifiers are egg yolk (where the main emulsifying chemical is lecithin), honey, and mustard, where a variety of chemicals in the mucilage surrounding the seed hull act as emulsifiers; proteins and low-molecular weight emulsifiers are common as well. So lecithin is another emulsifier and thickener. In some cases, particles can stabilize emulsions as well through a mechanism called Pickering stabilization. Both mayonnaise and Hollandaise sauce are oil-in-water emulsions that are stabilized with egg yolk lecithin.
They will physically interact with both oil and water, thus stabilizing the interface between oil or water droplets.
Common examples include emulsifying wax, cetearyl alcohol, polysorbate 20, and ceteareth 20. Sometimes the inner phase itself can act as an emulsifier, and the result is nanoemulsion - the inner state disperses into nano-size droplets within the outer phase. A well-known example of this phenomenon, when water is poured in a strong alcoholic anise-based beverage, The anisolic compounds, which are soluble in ethanol, now form nano-sized droplets and emulgate within the water. The colour of such diluted drink is opaque andmilky.
Microemulsions consist of large or ‘‘swollen’’ micelles, containing an internal phase similar to that found in a solubilized solution. Unlike macroemulsions, they appear as clear, transparent solutions. They tend to be more thermodynamically stable than macroemulsions and can have essentially infinite lifetimes assuming no change in composition, temperature and pressure. This is in contrast to macroemulsions which, although they may remain stable for long periods of time, will ultimately undergo phase separation to attain a minimum in free energy. Microemulsions can generally be obtained by gentle mixing of the ingredients of the emulsion. In this respect, they differ from macroemulsions which require intense agitation for their formation. Microemulsions are usually prepared with more than one surfactant or using a mixture of surfactant and cosurfactant (e.g., a polar compound of intermediate chain length).
Microemulsions have been studied as drug delivery systems, in particular for topical and transdermal drug delivery.[1,2,7]
SELECTION OF EMULSIFYING AGENT DEPENDING ON ITS HLB VALUE
HLB System
Griffin devised an arbitrary scale of values to serve as a measure of the Hydrophile - Lipophile Balance (HLB) of surface active agents. HLB is the ratio of oil soluble and water-soluble portions of a molecule. HLB value is increased with increased hydrophilic nature, and decreased hydrophobic nature of surfactant. These scales have optimum efficiency for each class of surfactant, as seen in figure. Values are assigned based upon that table ranging from 0 to 20.[19]

Fig. No.15.Scale Showing Surfactant Function On Basis Of HLB Values.
The HLB of non-ionic surfactant whose only hydrophilic portion is polyoxyethylene is calculated using the formula [5]
HLB = E/5
Where, E = the percent by weight of ethylene oxide.
A no. of polyhydric alcohol fatty acid esters, such as glycerylmonostearate, may be estimated by using formula[5]
HLB = 20(1-S/A)
Where,
S = saponification no. of ester,
A = acid no. of fatty acid,
The HLB of polyoxyethylene sorbitan monolaurate (Tween-20) for which S = 45.5 & A = 276
HLB = 20 (1-45.5/276) = 16.7
|
Sr. No. |
Name of Surfactant |
HLB Value |
Applications |
|
1 |
Acacia |
8 |
O/W Emulsifying Agent |
|
2 |
Glyceryl Monostearate |
3.8 |
W/O Emulsifying Agent |
|
3 |
Sorbitol monostearate |
4.7 |
W/O Emulsifying Agent |
|
4 |
Polysorbate 20 |
16.7 |
Solublizing Agent |
|
5 |
Polysorbate 60 |
14.9 |
Detergent |
|
6 |
Polysorbate 80 |
15 |
Solublizing Agent |
|
7 |
Sodium Lauryl sulphate |
40 |
Suspending Agent |
|
8 |
Sodium oleate |
18 |
Solublizing Agent |
|
9 |
Tragacanth |
13.2 |
O/W Emulsifying Agent |
|
10 |
Triethanolamine oleate |
12 |
O/W Emulsifying Agent |
Table. No.3. Some HLB Values And Their Use Of Surfactant [27]
II. Formulation of Aerosols
Surfactants are found in both solution and suspension formulations of metered dose inhalers (MDIs). The most common surfactants found in pressurised aerosol preparations include sorbitan trioleate (Span 85), oleic acid, and lecithins at concentrations of 0.1–2.0% (w/w). These agents are non-volatile liquids which dissolve in the propellant blend. Their function in the formulation is to provide lubrication for the metering valves and, in the case of suspension formulations, to maintain the disperse nature of the drug. The three surfactants commonly used in chloroflurocarbon (CFC)-based MDI formulations are insoluble in the CFC-replacement propellants, hydrofluoroalkane (HFA) 134a and HFA 227. Possible formulation alternatives involve the use of an adjuvant such as ethanol to aid dissolution of the surfactant or a novel surfactant. Several companies have investigated novel materials among which are fluorosurfactants, polyoxyethylenes and drugs coated with surfactant.[2,7]
* SEMISOLID SYSTEMS
Surfactants are major constituents of pharmaceutical, cosmetic, and food semisolid formulations, many of which are emulsions, either oil in water (o/w) or water in oil (w/o). They are included for their stabilizing, wetting, solubilizing, detergent and penetrationenhancing properties.
Water-in-oil emulsions traditionally contain surfactants of natural origin such as cholesterol, wool fat, wool alcohols, lanolin, divalent salts of fatty acids soaps, calcium oleate and/or synthetic agents of low hydrophilic-lipophilic balance (HLB) (indicating high lipophilicity), such as Spans (fatty acid esters of sorbitan).
An example of such a product is Oily Cream B.P. which consists of a 1 : 1 mixture of wool alcohols and water. Oil-in-water creams, for topical use, generally contain mixed emulsifiers/surfactants; one of which is a water soluble surfactant with a high HLB, the other being an amphiphile, usually a long chain fatty alcohol (e.g., of chain length C14 to C18) or acid (e.g., palmitic or stearic). The water soluble surfactant may be anionic (e.g., sodium lauryl sulphate), cationic (e.g., cetrimide), or non-ionic (e.g., cetomacrogol, Tweens). These mixed-surfactant systems are used not only for their ability to form complex condensed films at the liquid–liquid interface, enhancing the stability of the emulsion, but also because of their ability to impart ‘‘body’’ to the product, resulting in a semisolid product rather than a liquid. Mixed emulsifiers control the consistency of a cream by forming a viscoelastic network throughout the continuous phase of the emulsion. The network results from the interaction of the mixed emulsifier with water, forming a liquid crystalline phase.[1,10]
I. Formulation of Ointments
Ointments are semisolid preparation meant for external application to skin or mucous membrane; they usually contain medicaments or medicaments in dissolved, suspended or emulsified in an ointment base. Sometimes in the ointment preparation surfactants are useful for the easy removal from the skin by washing with water & also for the consistency by reduction of surface tension.
Surfactants are also used in formulation of cold cream, cleansing cream, vanishing cream, shaving cream or any media.[2]
II. Formulation of Shampoo
Shampoo is a hair care product used for the removal of oils, dirt, skin particles, dandruff, environmental pollutants and other contaminant particles that gradually build up in hair. The goal is to remove the unwanted build-up without stripping out so much as to make hair unmanageable.
Shampoo, when lathered with water, is a surfactant, which, while cleaning the hair and scalp, can remove the natural oils (sebum) which lubricate the hair shaft.[2,9]
* SOLID DOSAGE FORMS
Surface-active agents have been widely shown to enhance drug dissolution rates. This may be due to wetting effects, resulting in increased surface area, effects on solubility and effective diffusion coefficient or a combination of effects. Consequently surfactants have been included in tablet and capsule formulations to improve wetting and deaggregation of drug particles and thus increase the surface area of particles available for dissolution. This wetting effect is found to be operative at concentrations below the CMC. The effect of surfactants on the dissolution of solids is complex. In addition to effects on the available surface area, surfactants in concentrations above the CMC can increase drug solubility and hence the effective concentration gradient.
However they also reduce the effective rate of drug diffusion as a consequence of drug solubilization within micelles. Models to quantify the effect of surfactant concentration on drug dissolution have been developed. For solids whose dissolution is under significant surface control, surfactants may further influence the dissolution process. In this regard the enhancing effect of surfactants on the dissolution rate of cholesterol has been widely studied.
I. Hard Gelatin Capsules and Tablets
Wetting agents Surfactants are used in capsule and tablet formulations as wetting agents to aid dissolution.
Lubricants, anti-adherents, and glidants.
The primary function of tablet lubricants is to reduce the friction arising at the interface of tablet and die walls during compression and ejection. Lubricants also possess antiadherent (prevention of sticking to the punch and, to a lesser extent, to the die wall) and glidant (improvement of flow characteristics of powders or granulates) characteristics and are useful in the processing of hard gelatin capsules. Magnesium stearate is used extensively as a lubricant in tablet manufacture. It is an example of a ‘‘boundary lubricant,’’ that is, the polar regions of the molecule adhere to the metal surface of the die wall (in tablet manufacture). Adsorption of magnesium stearate to the powder or granule surfaces also prevents agglomeration of the feed material and aids flow. Lubricants may be classified as water-soluble or water-insoluble. The latter are generally more effective than water-soluble lubricants and can be used at a lower concentration. Common water-insoluble lubricants (which are surfactants) include magnesium stearate, calcium stearate, sodium stearate, and stearic acid; water-soluble lubricants include sodium lauryl sulphate and magnesium lauryl sulphate. Sodium lauryl sulphate is used in the production of hard gelatin capsules where it is added to the gelatin solution during the preparation stage. The stainless steel molds are lubricated prior to dipping into the gelatin solution and sodium lauryl sulphate is added to reduce the surface tension of the mix and cause the mold pins to wet more uniformly.[2,9]
II. Suppositories
Several non-ionic surface-active materials have been developed as suppositories vehicles. Many of these bases, known as water-dispersible bases, can be used for the formulation of both water-soluble and oilsoluble drugs. The surfactants most commonly used are thepolyoxyethylene sorbitan fatty acid esters (Tweens), the polyoxyethylene stearates, and the sorbitan fatty acidesters (Spans). These surfactants may be used alone, blended, or with other suppository base materials to yield a wide range of melting points and consistencies.
Surface-active agents are widely used in combination with other suppository bases. The inclusion of these agents in the formulation may improve the wetting and water-absorption properties of the suppository. In addition, emulsifying surfactants help to keep insoluble substances suspended in a fatty base suppository. The inclusion of a surfactant in the suppository formulation may enhance the rectal absorption of drugs. The effect has been attributed to the formation of mixed micelles. It has been suggested that the presence of the micelle facilitates the incorporation of the lipid component of the mixed micelle into the biological membrane. This lipid then enhances the fluidity and permeability of the membrane to the poorly absorbed drug. It appears that the colorectal mucous membrane is more sensitive to the effects of mixed micelles than the gastrointestinal membrane of the small intestine.[2]
DIRECT ACTIONS OF SURFACTANTS
a. Surfactant as Detergent
The term detergent is sometimes used to refer to any surfactant, even when it is not used for cleaning. This terminology should be avoided as long as the term surfactant itself is available. A detergent is a material intended to assist cleaning. The term is sometimes used to differentiate between soap and other surfactants used for cleaning. As an adjective pertaining to a substance, it (or "detersive") means "cleaning" or "having cleaning properties"; "detergency" indicates presence or degree of cleaning property.[2,19]
b. Foamer/Defoamer
A foaming agent is a surfactant, which when present in small amounts, facilitates the formation of foam, or enhances its colloidal stability by inhibiting the coalescence of bubbles. Sodium laureth sulfate, or sodium lauryl ether sulfate (SLES), is a detergent and surfactant found in many personal care products (soaps, shampoos, toothpaste etc.). It is an inexpensive and very effective foamer. Sodium lauryl sulfate (also known as sodium dodecyl sulfate or SDS) and ammonium lauryl sulfate (ALS) are commonly used alternatives to SLES in consumer products. While SLS is a known irritant, some evidence and research suggest that SLES can also cause irritation after extended exposure. Also, a foaming agent is a material that will decompose to release a gas under certain conditions (typically high temperature), which can be used to turn a liquid into a foam. For example, powdered titanium hydride is used as a foaming agent in the production of metal foams, as it decomposes to form titanium and hydrogen gas at elevated temperatures. Zirconium(II) hydride is used for the same purpose.
Defoamer or an anti-foaming agent is a chemical additive that reduces and hinders the formation of foam in industrial process liquids. The terms anti-foam agent and defoamer are often used interchangeably. A defoamer is normally used in industrial processes to increase speed and reduce other problems. It addresses both problems with surface foam and entrained or entrapped air. A wide variety of chemical formulas are available to promote coalescence of foam.Generally a defoamer is insoluble in the foaming medium and has surface active properties. An essential feature of a defoamer product is a low viscosity and a facility to spread rapidly on foamy surfaces. It has affinity to the air-liquid surface where it destabilizes the foam lamellas. This causes rupture of the air bubbles and breakdown of surface foam. Entrained air bubbles are agglomerated, and the larger bubbles rise to the surface of the bulk liquid more quickly.[2,6,9]
c. Antimicrobial Activity
Significant antimicrobial effects have been associated with cationic surfactants, in particular the quaternary compounds. The action mechanism of quaternary surfactants involves disruption of the cell membrane, protein denaturation, and enzyme inhibition. Quaternary compounds are able to lyse cells at relatively low concentration, resulting in leakage of cell contents into the surrounding medium. Quaternary ammonium and some phosphonium surfactants are used as topical disinfectants in commercial dermatological products, in surgical hand scrubs, and in the irrigation of skin wounds. The most commonly used quaternary compounds employed for theirantimicrobial effects are cetylpyridinium chloride, benzalkonium chloride, benzethonium chloride and cetyltrimethylammonium bromide. Other surfactants, containing more than one quaternary (or positively ionizable group) are among the most active substances known in terms of antimicrobial activity. Included in this group are dequalinium acetate and chlorhexidine gluconate which have been used in throat lozenges and mouthwashes. The lysis of cells can also occur in the presence of anionic surfactants, although these are in general weaker in their antimicrobial activity.
A wide range of anionics, in particular sodium lauryl sulphate and its homologs, finds wide application in mouthwashes.[28]
d. Surfactants as Laxatives & Spermicides
Disodium octyl sulfosuccinate the long term use is laxative. The mechanism of this action has not been fully explained.
Certain non-ionic ethers or nonyl- & octyl phenol are widely used as spermicides in conc. ranging as high as about 5%. The spermicidal action of these non-ionic ethers probably by their ability to distrup vible membranes or ability to dissociate lipids from lipoproteins. Since mucous membrane of vaginal membrane are not damaged by presence of nonoxynols or octoxynols.[2,28]
REFERENCES
1. Abramzom AA. REVIEWS-SURFACTANTS THEIR PROPERTIES AND USE Translated from Khtmiko-Farmatsevticheskit Zhurnal, Moscow. [serial online] 1977 [2009 November 08]; Vol. 11(1):149-150.
2. Corrigan OI, Healy AM. Surfactants in Pharmaceutical Products and Systems. In: Swarbrick James, editors. Encyclopedia Pharmaceutical Technology. 3rd ed. NY:Informa Healthcare USA, Inc. 270 Madison Avenue; 2007. vol 1. p. 3583-97.
3. Rawlins EA, editors. Bentley’s Text book of Pharmaceutics. 8th ed. IND: All India Traveller Book Seller, Delhi; 2009. p. 33, 44,342,501-6.
4. Liberman Herbert A, Rieger Mattin M and Banker Gilberts, Editors. Pharmaceutical dosage forms: Disperse system. 2nd ed, Revised and Expanded. NY: Banker series; Vol. 1. p. 389. ------22
5. Martin Alfred. Physical Pharmacy-Physical Chemical principles in Pharmaceutical Sciences. 4thed. P. 362-392.
6. Perkins Warren S. Surfactants -A Primer An in - depth discussion of the behavior of common types of surfactants. In: Dyeing, Printing & Finishing. 1998;51.
7. Chemical Functional Definitions.[online]. URL: chemistry.co.nz/surfactants.htm [2009 October 08].
8. Chevalier Y., Zemb T., The structure of micelles and microemulsions. Rep Prog Phys, 1990; 53:279-371.
9. Surfactant - Wikipedia, the free encyclopedia.[online]. URL:en.wikipedia.org/wiki/Surfactant [2009 Oct 08].
10. Bhargava H, Narurkarr A. Using microemulsion for drug delivery. Harm Tech. 1987; 12,45,46.
11. Tanford C. The hydrophobic effect: Formation of micelles and biological membranes. Wiley. NY; 1980:3-8.
12. Atwood D, Florence AT. Surfactant system. Champman and Hall: London; 1983.
13. Faeder J, Ladanyi B, Molecular Dynamics Simulations of the Interior of Aqueous Reverse Micelles. A Chem Soci;1984:13-9
14. Keir RI, Watson JN, Stradner A. Micellisation of metal alkanoates in non-aqueous media. Coll. Surf. A: 1999;157- 203.
15. Zingaretti L., Boscatto L., Chiacchiera M., Silber J., Kinetics and mechanism for the reaction of 1-chloro-2,4-dinitrobenzene with n-butylamine and piperidine in AOT/n-hexane/water reverse micelles. ARKIVOC 2003; 34:189-200.
16. Yagui C, Junior A, Tavares L. Micellar solubilization of drugs. J Pharma, Pharmaceutical Sci. 2005;8(2);147-163.
17. Malmsten M. “Surfactants and polymers in drug delivery system”, Marcel Dekker, Inc., 2002, Page no.9-27.
18. Kwon GS, Kataoka K. Block copolymer micelles as long circulating drug vehicles. Adv Drug Deliv Rev.1995;16:295-309.
19. Loyd V. Allen, Jr. Nicholas G. Popovich, Howard C. Ansel, “Ansel’s Pharmaceutical Dosage form and Drug Delivery System”, Eighth Edition 2005, 250, 385-442
20. Yalkowski, Samuel H., Solubility of Organic Solutes in Mixed Aqueous Solvent, Final Report to the R. S. Kerr Research Lab., U.S. EPA, contract CR811852-01-0, 1985.
21. Lachaman, Lieberman, Kanig, “The Theory and Practical of Industrial Pharmacy”, Third edition, 479-563.
22. Liberman Herbert A, Rieger Mattin M and Banker Gilberts, Editors. Pharmaceutical dosage forms: Disperse system. 2nd edition revised and expanded. NY: Banker series; Vol. 1. p. 389.
23. URL: researchd.com/rdioem/surfkt.htm[2009 Nov 20]
24. Liberman Herbert A, Rieger Mattin M and Banker Gilberts, Editors. Pharmaceutical dosage forms: Disperse system. 3rd ed, Revised and Expanded. NY: Banker series; Vol. 3. p. 430.
25. Allen Loyd V, Jr. Popovich Nicholas G, Ansel Howard C. Ansel’s Pharmaceutical Dosage form and Drug Delivery System. 8th ed. India: Wolters Kluwer Pvt. Ltd, New Delhi.2005; 250,385-442.
26. Avis Kenneth E, Lachman Leon and Liberman HA, Editors. Pharmaceutical Dosage forms: Parentral Medications. NY: Dekker, INC; vol.1. P.152-5.
27. Liberman Herbert A, Rieger Mattin M and Banker Gilberts, Editors. Pharmaceutical dosage forms: Disperse system. 3rd Ed, Revised and Expanded. NY: Banker series; Vol. 2. p. 32,48, 52-5.
28. URL:dermatology.about.com/od/glossarys/g/surfactant.htm[2009 Nov 13]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









