About Authors:
*Sunil kumar, Amit kumar shahi, Ravi shanker, R. singh, Dr. R. ParthSarthy, Dr S.K. Prajapati
Kamla Nehru institute of technology and management, Sultanpur.
Bundelkhand university, Department of pharmacy, Jhansi
*sunil.sunilpharma.kumar@gmail.com
ABSTRACT
The purpose of this research is to design proniosomal powder drug delivery system of flurbiprofen in a trial to overcome the adverse effects associated with oral administration of the drug. Conventional chemotherapy for the treatment of intracellular infection is no more effective due to limited permeation of drug into cell. This can be overcome by the use of vesicular drug delivery system. Encapsulation of a drug in vesicular structure can be predicted to prolong the existence of the drug in the systemic circulation and thus enhance penetration into target tissue and reduce toxicity.Proniosomal powder are generally present in transparent, translucent or white texture, which makes them physically stable during storage and transport. Due to the limited solvent system present, the proniosomes formed were the mixture of many phases of liquid crystal, viz. lamellar, hexagonal and cubic phase liquid crystals.The potential of proniosomes as a transdermal drug delivery system for flurbiprofenwas investigated by encapsulating the drug in various formulations of proniosomal powder composed of various ratios of sorbitan fatty acid esters, cholesterol, prepared by slurry method. The formulated systems were characterized in vitro for size, vesicle count, drug entrapment, drug release profiles and vesicular stability at different storage conditions. Stability studies for proniosomal powder were carried out for 4 weeks. The method of proniosome loading resulted in an encapsulation yield of 30.6 – 75.4%. Proniosomes were characterised by transmission electron microscopy. In vitro studies showed prolonged release of entrapped flurbiprofen. At refrigerated conditions, higher drug retention was observed. It is evident from this study that proniosomes are a promising prolonged delivery system for captopril and have reasonably good stability characteristics.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1322
INTRODUCTION
Non-ionic surfactant vesicles known as niosomes are microscopic lamellar structures formed on admixture of a non-ionic surfactant, cholesterol and dicetyl phosphate with subsequent hydration in aqueous media. Proniosomes offer a versatile vesicle drug delivery concept with potential for delivery of drugs via transdermal route. This would be possible if proniosomes form niosomes upon hydration with water from skin following topical application under occlusive conditions. Proniosomes minimizes problems of niosomes physical stability such as aggregation, fusion and leaking and provide additional convenience in transportation, storage and dosing. Transdermal therapeutic system has generated an interest as this system provides the considerable advantage of a non-invasive parenteral route for drug therapy, avoidance of first pass gut and hepatic metabolism, decreased side effects and relative ease of drug input termination in problematic cases.Provesicular systems had attracted researchers as an alternate strategy for transdermal delivery of drugs because of the non-toxicity and penetration effect of lecithin/surfactants. Provesicular systems have been exploited in oral drug delivery in the form of tablets, beads or capsules and have shown improved dissolution and absorption characteristics. Based on the investigations provesicular systems appear to be an alternate drug carrier for various routes of drug administration.Flurbiprofen, are nonsteroidal anti-inflammatory drug (NSAIDs) is used for the relief of pain and inflammation associated with rheumatoid arthritis and osteoarthritis. It exhibits anti-inflammatory, analgesic and antipyretic activities. It is also used in mild to moderated pain including dysmenorrheal and migraine. Flurbiprofen is more potent than Ibuprofen but has more gastric side effectlike peptic ulceration and severe gastrointestinal bleeding may occurs. This drug may also cause serious effect on stomach or intestine including bleeding or perforation (forming of hole). The plasma half life (t1/2) of flurbiprofen is 4-6 hours. Hence repeated administration of high dose (100 mg: three time a day) is required for effective management of rheumatoid arthritis and osteoarthritis.It will be also effected the transdermal system rate because of its size, nature and chemistry, these systems give better drug permeability from biological bioavailability membranes and helps in solubilization of some practically insoluble drugs and hence solve problems of many drug.
To overcome the problem like gastric side effect, short half life and low bioavailability etc of flurbiprofen can be solved by developing the formulation of flurbiprofen as proniosome carriers in the form powder.
Materials:
Flurbiprofen was a gift from F.D.C. Mumbai, cholesterol, and dialysis tubing were purchased from Hi-Media Laboratories (Mumbai, India). Span 20, 40, 60, 80 and BRIJ 35 were purchased from Central Drug House (Delhi, India).All other chemicals and solvents were of annular grade and obtained from C.D.H Company for pharmaceutical chemicals,(Delhi, India).
[adsense:468x15:2204050025]
Method:
Proniosomes were prepared by Slurry method.
Strategies for the preparation of provesicles in the preparation of proniosomes non-ionic surfactants, coating carriers and membrane stabilizers are commonly used. The non-ionic surfactants used are Span (20, 40, 60, 80, Brij 35). The coating carriers used is maltodextrin (Maltrin M500, M700), membrane stabilizers like cholesterol are also used.
For ease of preparation a stock solution of accurately weighted quantities of surfactant, cholesterol and drug was prepared in 10 ml chloroform: methanol (2:1) solution the required volume of surfactant, cholesterol stock solution and drug was added to a 100 ml round bottom flask containing 500 mg maltodextrin carriers. Additional chloroform: methanol solution was added to form slurry in the case of lower surfactant loading. The flask was attached to a rotary evaporator to evaporate solvent at 60-70 rpm, a temperature of 45±2ºc and a reduced pressure of 600 mmhg. Until the mass in the flask had become a dry free flowing product. These materials were further dried overnight in desecator under vaccum at room temperature. This dry preparation is referred to as Proniosomes Powder.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Characterization of Proniosomal Powder
Optical microscopic examination:
Hydration of proniosomal gel (100mg) was done by adding saline solution (0.9% solution) in a small glass vial with occasional shaking for 10 min. The dispersion was observed under optical microscope at 100 x magnification. The sizes of 200-300 vesicles were measured using a calibrated ocular and stage micrometer (Erma, Tokyo) fitted in the optical microscope.
Vesicle Size Analysis:
Hydration of proniosomal gel (100mg) was done by adding saline solution (0.9% solution) in a small glass vial with occasional shaking for 10 min. The dispersion was observed under optical microscope (Olympus, New Delhi) at 100 x magnification. The sizes of 200-300 vesicles were measured using a calibrated ocular and stage micrometer (Erma, Tokyo) fitted in the optical microscope
Entrapment efficiency of proniosomal powder
To evaluate the loading capacity of proniosomal systems for flurbiprofen, proniosomal powder (100mg) was dispersed in distilled water and warmed a little for the formation of niosomes. Then the dispersion was centrifuged at 18000 rpm for 40min at 5ºC (Remi CPR-24 centrifuge). The clear fraction was used for the determination of free drug at 247.0 nm spectrophotometrically. The percentage encapsulation efficiency was calculated from Equation 1...
% Encapsulation Efficiency = [1- (Unencapsulated drug / Total drug)] x 100
In-Vitro Drug Release of proniosomal powder
The in vitro drug release studies were carried out by means of treated dialysis membrane.
The release rate of Flurbiprofen from proniosomal powder was carried out in using dialysis bag method.
A measured amount of niosome suspension equivalent to 5.5 mg were placed dialysis bag of effective length 8 cm. Dialysis bag was placed in a beaker containing 500 ml of simulated gastric fluid. The beaker was placed over magnetic stirrer having stirring speed of 100 rpm.The temperature of medium was maintained at 37ºC by a thermostatic control available on the magnetic stirrer. Aliquots of sample (5 ml) were withdrawn periodically and replaced with the same volume of fresh fluid, at each sampling point. The samples withdrawn were analyzed for the drug content at 247 nm spectrophotometrically. The same is also repeated for phosphate buffer saline of pH 7.4, and volume of 500 ml. the sample withdrawn were analyzed for the drug content at 247 nm spectrophotometrically. All the determination was made in three times.
Stability Studies:
The ability of vesicles to retain the drug (Drug Retention Behaviour) was assessed by keeping the proniosomal powder at three different temperature conditions, i.e., Refrigeration Temperature (4-80C), Room Temperature (25±20C) and oven (45±20C). Throughout the study, proniosomal formulations were stored in aluminium foil-sealed glass vials. The samples were withdrawn at njdifferent time intervals over a period of one month and drug leakage from the formulations was analyzed for drug content spectrophotometrically.
RESULTS AND DISCUSSION
Drug entrapment within a vesicular carrier is an important parameter to be defined to really evaluate the delivery potentiality of the system. For this reason, the entrapment efficiency of flurbiprofen within the formulations varies from as low as varies from 55.3% for Span 80 vesicle (PQR3) to high as 75.42% for Brij 35 vesicle Table 7.21, high entrapment efficiency for span 60 formulation can be attributed to itslength of longer side chain, and it easily diffuses into the receptor fluid membrane integrity, orientation and packaging ability.
The proniosomal formulation having low cholesterol content was found to cause low entrapment efficiency, which might be because of leakage of vesicle. The higher entrapment may be explained by high cholesterol content (50 % of the total lipid) Table 7.21 reported that entrapment efficiency increase with increasing cholesterol content. It was also observed that very high cholesterol content had a lowering effect on drug entrapment to the vesicle. This could be due to the fact that cholesterol beyond a certain level starts disrupting the regular bilayer structure leading to loss of drug entrapment.
In-Vitro Drug Release Studies through Dialysis Membrane
The release study was conducted for all the optimized formulation (formulation showing better entrapment efficiency, optimum vesicle size, Spherical surface morphology). Most of the formulation were found to have a linear release and the formulation (SKT3) was found to provide approximately 58.23% release with in a period of 12 hrs. The amount of drug release from different proniosomal powder formulation was found in order of SKT3 > SKS3 > SKP3 > SKR3 > SKP3. Table 7.29, from the release profile, it appears that flurbiprofen efflux from niosome is a process containing a slower release phase achieved with in 2-4 hr. in solution. In first hour rapid release, and after 10 hour, slow release occur, in which 5-10% of flurbiprofen was lost from different proniosomal preparation this could be because of the drug is mainly incorporated between the fatty acid chain in the lipid bilayer of niosome vesicle, which leads to rapid ionization and release upon dispersing niosome in larger buffer (pH 7.4), volume until reaching equilibrium. This result is in accordance with increasing cholesterol beyond a certain concentration can disrupt the regular linear structure of the vesicular membrane and increase the drug release. Fig. 7.40 illustrates the drug release profiles from its free solution and different proniosomal formulation. The release data were analyzed mathematically according to zero-order, first order, and Higuchi equations. From the study it was found that formulation SKT3 exhibited satisfactory results, hence considered as better formulation. In order to ascertain release kinetics, the rate constant for zero order, first order and Higuche equation kinetics were calculated for each time interval, the release constant was calculated from the slope of appropriate plots, and the regression coefficient (r2) was determined. It was found that the in-vitro drug release of proniosome was best explained by first order kinetics for best formulation SKT3 as the plots show highest linearity. The correlation coefficient (r2) was found 0.96. It is satisfactory release kinetics of selected formulation. The best formulation SKT3 was found to give a cumulative release of 58.32% over a period of 24 hrs, Table 7.29. Same release pattern was observed either in the case of simulated gastric fluid or phosphate buffer saline.
Kinetic Data Treatment
To find out the kinetics and mechanism of drug released from all the formulations of flurbiprofen encapsulated proniosomes, In the case of proniosomal powder the data were treated according to zero order, first order and Higuchi’s equation pattern. As the data clearly indicated in the Table 7.30 to 7.32, the correlation coefficient of the formulation (SKT3) was found 0.901 in zero order equation pattern when the data were plotted according to first order equation, the correlation coefficient was found to be 0.965, and in Higuachi equation the correlation coefficient found to be 0.938. Hence the formulation (SKT3) follows first order equation.
Table: 2 various result of proniosomal gel formulation
|
S. No. |
F. Code |
Vesicle size (nm) |
% Entrapement efficiency |
% Amount drug released |
|
1. |
SKP3 |
498.7 |
55.3±1.97 |
45.43±0.45 |
|
2. |
SKQ3 |
376.4 |
59.3±1.65 |
47.12±0.87 |
|
3. |
SKR3 |
305.3 |
70.1±1.98 |
44.32±0.86 |
|
4. |
SKS3 |
204.3 |
56.0±1.63 |
42.45±0.56 |
|
5. |
SKT3 |
275.7 |
75.4±1.55 |
58.53±0.67 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
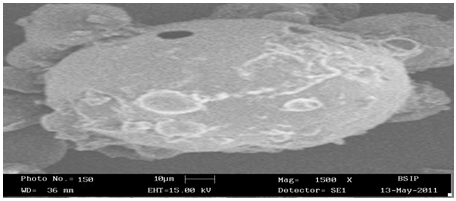
Scanning electron microscopy examination of proniosome derived niosome (SKT3)
The effect of cholesterol on flurbiprofen entrapment was varied according to the nonionic surfactant used, cholesterol was found to have little effect on the flurbiprofen entrapment was obtained when 10% of cholesterol was incorporated into niosome, prepared from span 40 & span 60, followed by decrease in encapsulation efficiency of the drug upon further increase in cholesterol content. The two factor further increase in cholesterol on Entrapement one is with increasing cholesterol, the bilayer hydrophobicity, and stability increased and permeability decreased which lead to efficiently trapping the hydrophobic drug into bilayer as vesicle formed, other factor stated that higher amount of cholesterol may compose with drug for packing space with in the bilayer hence excluding the drug as the amphiphiles assemble into vesicle, other study suggested that increasing cholesterol ratio above certain limit may be can disrupt the regular linear structure of vesicular membranes.(Pardakhty et al. 2006). The proniosomal formulation having low cholesterol content was found to cause low entrapment efficiency, which might be because of leakage of vesicle. The higher entrapment may be explained by high cholesterol content (50 % of the total lipid) Table 7.24 reported that entrapment efficiency increase with increasing cholesterol content. It was also observed that very high cholesterol content had a lowering effect on drug entrapment to the vesicle. This could be due to the fact that cholesterol beyond a certain level starts disrupting the regular bilayer structure leading to loss of drug entrapment. It is shown in Table 7.24 (Abdelbary etal. 2008)
This result are also observed and similar as study in the proniosomal formulation using Brij 35, span 60 and tween 80 surfactant. In this prepare vesicle using Brij 35: cholesterol show better drug release in comparision to other surfactant. (Abdelbary etal. 2008). Our finding results are observed similar as study in Brij 35, Brij 58, and Brij 92 Brij 52 etc. The conclusion of this stydy show that Brij 35 did not form niosome in the absence of cholesterol, but in the presence of cholesterol niosome formed and vesicle shows more stable and less leaky. (Pardakhty et al. 2006)
This result are also observed in a comparative study on the effect of some polyoxyethylene alkyl eather like (Brij 78, Brij 92, Brij 72, Brij 52) and sorbitan fatty acid ester surfactants on the performance of transdermal proniosomal gel using experimental design. In this the Brij 52 show similar property as compare to Brij 35.The findings of this study shows that the niosome formulation prepared with Brij 52 was better drug release in comparasion to other Brij Component. (Aboelwafa et al. 2010)
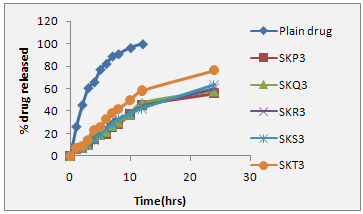
Figure: 1 In-vitro drug release profile of selected proniosomal gel formulation
On the study of many research paper and also included my research work, most of the surfactant used to make non-ionic surfactant vesicle have low aqueous solubility, however freely soluble non-ionic surfactant such as Brij 35 can form micelles on hydration due to the presence of more polar head group in the chain, in the addition of cholesterol they abolish the more polar part present in surfactant mainly due to lipophillic in nature and help in formation of vesicle in the equmolar ratio of Brij 35 and cholesterol show better result. The proniosome formation takes place from Brij 35 with the presence of cholesterol, the length of alkyl chain show a crucial factor of permeability, Brij 35 have long lauryl(c12) chain, thus the long chain influence the HLB of the surfactant and also lead to the higher drug entrapment efficiency and also show better stability of the proniosome using Brij 35. Cholesterol is one of the most important additives included in the formulation in order to prepare stable niosome. Cholesterol stabilize bilayer, prevents leakiness and retards permeation of solutes enclosed in the aqueous core of these vesicle. Cholesterol is known abolish the gel to lipid phase transition of niosome system, which could be able to effectively prevent leakage of drug from niosome. Cholesterol is thus included in 1: 1 molar ratio (non-ionic surfactant: cholesterol), show better result in the case of Brij 35. Thus it can be concluded that Brij 35 show highest drug release by the fact that niosome exhibt an alkyl chain length-dependent drug release
Drug Leakage Studies
In order to determine the percent drug remaining entrapped in vesicles and percent drug lost from Proniosome powder subjecting at temperature 4±2oC, 37±2oC and 45±2oC for 45 days, were determined drug lost at time interval of 15 days. On the basis of entrapment efficiency and controlled release property, formulation SKT3 were selected for the stability studies. Stability study was carried out in term of % drug release.
Results showed that proniosomal powder formulation was quite stable at refrigeration and room temperature. In this condition not much leakage of drug was found at there temperature. Percent drug retained at 45ºC might have decreased due to the melting of surfactant and lipid present in the formulation to the proniosomal powder formulation can be stored at refrigeration and room temperature. Thus it can be concluded that the shelf life of proniosomal powder formulation is more than the proniosomal formulation. Because in dry surfactant can be avoided, by forming the suspension as needed, precipitation and aggregation can also be avoided.
Conclusion
Thus it can be concluded that the proniosomes powder posses higher entrapment efficiency and utilizes alcohol, which itself act as penetration enhancer. The elicited an increase of the percutaneous permeation of flurbiprofen in-vitro and. In addition, In-vitro experiments showed that flurbiprofen proniosomes powder can ensure a sustained release of the drug and hence a prolongation of its therapeutic activity, which can be related to an accumulation of flurbiprofen in the skin.
These findings are very encouraging and confirm that proniosomes are a very promising carrier for the topical/oral administration due to the enhanced delivery of drugs through the skin thus prompting various opportunities for the development of suitable therapeutic strategies through the topical route. The formulation is easy to scale up as the procedure is simple and do not involve lengthy procedure and unnecessary use of pharmaceutically unacceptable additives. It offers direct fabrication of transdermal patch and do not require dispersion of vehicle into polymer matrix.
ACKNOWLEDGEMENT
The authors acknowledge the financial support received from my parents. F.D.C Pvt. Ltd. Mumbai. Kamla Nehru institute of technology and management Sultanpur Department of pharmacy. For their support and encouragement to carry out this work.
REFERENCES
1. Shahiwala A, Misra A. Studies in topical application of niosomally entrapped nimesulide. J Pharm Pharm Sci 2002; 5(3): 220-225.
2. Khandare JN, Madhavi G, Tamhankar BM. Niosomesnovel drug delivery system. East Pharm 1994; 37:61-64.
3. Fang JY, Yu SY, Wu PC, Huang YB, Tsai YH. In-vitro skin permeation of estradiol from variousproniosomes formulation. Int J Pharm 2001; 215: 91-99.
4. Hu C, Rhodes DG. Proniosomes: A Novel Drug Carrier Preparation. Int J Pharm 1999; 185(1): 23-35.
5. Wu PC, Huang YB, Chang JJF, Chang JS, Tsai YH. Evaluation of pharmacokinetics and
6. pharmacodynamics of captopril from transdermal hydrophilic gel in normotensive rabbit and
7. spontaneously hypertensive rats. Int J Pharm 2000; 209: 87-94.
8. Abubkar O Nur, Zhang JS. Recent progress in sustained/controlled oral delivery of captopril: An overview. Int J Pharm 2000; 194: 139-146.
9. Yoshioka T, Sternberg B, Florence A. T. Preparation and properties of vesicles (Niosomes) of sorbitan monoesters (Span- 20, Span- 40, Span- 60 and Span- 80) and a sorbitan triester (Span- 85). Int. J. Pharm. 1994, 105, 1-6
10. Lusia D. Marianecci C. Novel pH sensitive non-ionic surfactant vesicles: comparison between Tween 21 and Tween 20. colloids and surfaces B: Biointerfaces 2011,82, 18-24
11. Tangri P, Khurana S. Niosomes: Formulation and evaluation. International journal of biopharmaceutics 2011, 2(1), 47-53.
12. Singh J, Robinson DH. Controlled release kinetics of captopril form tableted microcapsules. Drug Dev Ind Pharm 1988; 14(4): 545-560.
13. Leung SHS, Robinson JR. The contribution of anionic polymer structure feature to mucoadhesion. J Contrl Rel 1988; 5: 223-231.
14. Sheth PR, Tossounian J. The hydrodynamically balanced system (HBSTM): A novel drug delivery system for oral use. Dev Ind Pharm. 1984; 10(2): 313-339.
15. Abdelbary .G, El-gendy N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech, 2008, 105 8-9105.
16. Pardakhty A, Varshosaz J. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. International Journal of Pharmaceutics 2007, 318, 130–141.
17. Zhou XH, Li Wan PA. Stability and in-vitro absorption of captopril, enalapril and lisinopril across the ratintestine. Biochem Pharmacol 1994; 47: 1121-1126.
18. Tripathi KD. Essentials of Medical Pharmacology. New Delhi, India, Jaypee Brothers, 2003 pp 449-454.
19. Jain NK, Vora B, Khopade AJ. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Rel 1998; 54: 149-165.
20. Nagarsenker MS, Londhe VY, Nadkarni GD. Preparation and evaluation of liposomal formulation of tropicamide for occular delivery. Int J Pharm 1999; 190: 63-71.
21. Bhatia A, Kumar R, Katare OP. Tamoxifen in topical liposomes: Develoment, characterization and invitro evaluation. J Pharm Pharm Sci 2004; 7(2): 525-259.
22. Joshi YM, Bachman WR, Jani NB. New pharmaceutical composition in the form of beadlets and method. European Patent 1988; EP 288732: A2.
Received on 8th Jun, 2012 | Published on 6th Jul, 2012
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









