About Authors:
Akash M Patel*, Jitul B Patel
*Faculty of Pharmacy, Dharmsinh Desai University,
Nadiad-387001, Gujarat
*aku.pharmacy@gmail.com
Abstract
The purpose of this research work to prepare agglomerate of naproxen sodium for enhanced dissolution and improved physicochemical properties. Spherical crystallization technique was used to prepare agglomerates of naproxen sodium. Solubility of naproxen sodium was determined in different solvents for find out good solvent and bad solvents. Agglomerates of naproxen sodium alone and with incorporation of different polymer were prepared and stirring rate was also optimized. Prepared agglomerates were characterized for particle size, in-vitro drug dissolution study, % compressibility and different tableting parameters. Prepared agglomerates were characterized by DSC, XRD, FT-IR, and SEM. Results of in-vitro dissolution study indicated that agglomerates prepared by spherical crystallization were having higher dissolution than the naproxen sodium alone. Incorporation of polymers like PEG 400 and PVP K30 was also improved tableting characteristics as well as in vitro dissolution than agglomerates without incorporation of polymers. In conclusion, agglomeration of poorly water soluble drugs like naproxen sodium by spherical crystallization technique was successfully implemented for dissolution enhancement.
REFERENCE ID: PHARMATUTOR-ART-1904
INTRODUCTION
Spherical crystallization is a particle design technique, by which crystallization and agglomeration can be carried out simultaneously in one step and which has been successfully utilized for improvement of flowability and compactability of crystalline drugs1-5. Presently, particle design techniques are widely used in pharmaceutical industries to modify primary properties like particle shape, size, crystal habit, crystal form, density, porosity etc. as well a secondary properties like flow ability , compressibility, compact ability, reduction In air entrapment,etc Spherical crystallization is one of such particle design technique in which crystallization and agglomeration process are carried out simultaneously. Spherical Crystallization process transforms the fine crystal obtains during crystallization into spherical agglomerates. Agglomerates formed further improves the flowability and compressibility of pharmaceutical ingredient which enables direct tabletting of drug instead of further processing like mixing , granulation, seiving, drying etc. There are certain parameters which have to be optimized in order to obtain the maximum amount of spherical crystals6-8.
Some advantages of the spherical crystallization technique are listed below:
1) Spherical crystallization technique has been successfully utilized for improving of flowability and compressibility of drug powder.
2) This technique could enable subsequent processes such as separation, filtration, drying etc to be carried out more efficiently.
3) By using this technique, physicochemical properties of pharmaceutical crystals are dramatically improved for pharmaceutical process i.e. milling, mixing and tabletting because of their excellent flowability and packability.
4) This technique may enable crystalline forms of a drug to be converted into different polymorphic form having better bioavailability.
5) For masking of the bitter taste of drug.
Spherical crystallization is a fast developing technique of particle design in which crystallization and agglomeration can be achieved simultaneously in one step. It has been successfully utilized for improvement of flow ability and compactability of crystalline drugs. The principle steps involved in the process of spherical crystallization are flocculation zone, zero growth zone, fast growth zone and constant size zone9-15. General methods of spherical crystallization are emulsion solvent diffusion, spherical agglomeration, ammonia diffusion method and neutralization method. Factors controlling the process of agglomeration are solubility profile, mode and intensity of agitation, temperature of the system and residence time16, 17. Characterization of spherical crystals can be carried out using Optical Microscopy, Scanning Electron Microscopy, X-ray Powder Diffraction, Fourier Transform Infrared spectroscopy, Differential Scanning Calorimetry and Thin layer Chromatography.
Naproxen sodium is a [(+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid], a non-steroidal anti-inflammatory drug (NSAID). It has been indicated for various painful indications and proved as effective as other NSAIDs with lower indications of gastro-intestinal adverse effects and thus, resulted in a greater compliance with treatment. Naproxen sodium is practically insoluble. Solubility of Naproxen Sodium is 0.00159 mg/ml. log P value of Naproxen Sodium is 3.29, which indicated lower solubility in water. For poorly soluble orally administered drugs, the rate of absorption is often controlled by the rate of dissolution.
Present investigation aims to enhance dissolution of Naproxen sodium. Naproxen sodium was poorly water soluble drug and has poor tableting properties. Agglomeration of Naproxen sodium with different polymers using spherical crystallization technique will provide agglomerates or spherical crystals have good aqueous solubility and tableting property. Agglomerates prepared using spherical agglomeration using PEG 400 and PVP K-30 showed higher drug dissolution and good flowability and compressibility.
MATERIALS AND METHODS
Naproxen Sodium was procured from Sun Pharmaceuticals Advanced Research Center, Vadodara, India as a gift sample. PEG 400 and PVP K-30 was purchased from BASF Ltd, Mumbai, India. All the chemicals and solvents used in the preparation were of analytical grade. All materials were used as they were received.
EXPERIMENTAL
Development of Spherical Naproxen Sodium Crystals by Spherical Agglomeration Method
In order to produce spherical agglomerate of Naproxen sodium, 5 g of sample was dissolved in methanol (100 ml) at 60°C to make quasi-saturated solution. The resultant solution (5% w/v) was poured into mixture of water (840 ml) and PEG 400 (0.5 ml) thermally controlled at temperature 20 °C, with stirring at 400 rpm. The precipitated crystals were collected after 20 min by vacuum filtration. The obtained crystals were dried in an oven at 60°C for 3 h. The dried crystals were stored in desiccators at room temperature before use18-20. The above process was repeated several times in order to obtain enough Naproxen sodium for flow and particle size analysis tests. Composition of different Batches of Spherical Agglomerates of Naproxen Sodium was depleted in Table 1.
Effect of polymer concentration
The polymer (PEG 400/PVP K-30) was dissolved along with poorly soluble drug in mixture of good solvent (methanol) and bridging liquid in the different polymer concentration. The solution was poured into specific volume of distilled water (poor solvent) with an optimized stirring rate (400rpm) using propeller type of agitator at room temperature21,22. After agitating the system for 30min, the prepared agglomerates were collected by filtration through Whatman filter paper no.1. The spherical crystal were washed with distilled water and placed at room temperature for drying for 24 h.
Table 1: different Batches of Spherical Agglomerates of Naproxen Sodium
|
Composition |
Batches |
||||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
|
|
Naproxen Sodium (mg) |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
|
Methanol(ml) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
PEG 400 (%W/V) |
1% |
2% |
3% |
4% |
5% |
- |
- |
- |
- |
|
PVP K 30 (% W/W) |
- |
- |
- |
- |
- |
2.50% |
5% |
7.50% |
10% |
|
Water(ml) |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
|
RPM |
200 |
200 |
200 |
200 |
200 |
200 |
200 |
200 |
200 |
Evaluation of spherical agglomeration
Angle of repose
This is the common method used for determination of flow property. The angle of repose is the angle between the horizontal and the slop of the heap or cone of solid dropped from some elevation. Values for angle of repose ≤ 30 usually indicate free flowing material and angle ≥ 40 suggested a poor flowing material23-25. The angle of repose can be obtained from equation
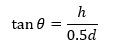
Where, h= height of the cone and d= diameter of the cone
% Compressibility
A simple indication of ease with which a material can be induced to flow is given by application of compressibility index26-28
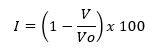
Where V = the volume occupied by a sample of powder after being subjected to a standardized tapping procedure and VO = the volume before tapping. The value below 15% indicates good flow characteristics and value above 25% indicate poor flowability.
Particle size determination
Particle size determination was carried out using optical microscopy with a calibrated eye piece micrometer and stage micrometer by taking a small quantity of formulation on slide. About 100 spherical agglomerates size was measured individually, average was taken and their size range and mean diameter frequency was calculated29, 30. Average Particle size is calculated by the formula,
∑nd
Average Particle size = --------
n
% Yield
It was determine by
%yield = Practical yield * 100
----------------------
Theoretical yield
Solubility study
Excess amounts of Naproxen Sodium agglomerates and pure Naproxen Sodium was dispersed in 20ml distilled water. The dispersion was shaken at 100rpm at 37°C for 24hrs using mechanical shaker. After 24hrs the dispersion was filtered through Whatman filter paper (grade1, circles, 90mm diameter), followed by dilution. The filtered sample solution was analyzed using a UV-visible spectrophotometer at 270nm. The mean results of triplicate measurements and the standard deviation were reported.
Drug content
Drug content was determined by dissolving 500 mg equivalent Naproxen Sodium in 100ml methanol and if necessary making further dilution using methanol31, 32. Amount of drug dissolved was analyzed spectrophotometrically at λmax of 270nm and drug content find out using calibration equation. All studies were carried out in triplicates.
In Vitro Dissolution Study
Accurately weighed SA of Naproxen sodium containing equivalent amount of Naproxen Sodium 500 mg was taken for in vitro dissolution study. In vitro dissolution study was carried out using USP dissolution apparatus type –II. 900 ml phosphate buffer pH 6.8 was taken as dissolution medium. Dissolution test was performed at 37ºC±5ºC. At regular time interval of 10 min 10 ml aliquots was withdrawn and fresh 10 ml dissolution medium was added to achieve sink condition. Sample withdrawn was analyzed at 270 nm using UV- spectrophotometer to calculate in vitro drug release (n=3).
Physical Characterization of Spherical Agglomerates
Differential Scanning Calorimetry (DSC) Study
A differential scanning calorimeter was used to monitor thermal events during heating. Samples weighing 2 -3 mg were placed in an open aluminum pans and heated from 55 -250°c at a rate of 10°c per min. Nitrogen was used as a purge gas at a flux rate of 50ml/min. The onsets of melting points and enthalpies of fusion were recorded by software provided by supplier.
Fourier Transform infrared spectroscopy Study
The FTIR spectra of Naproxen Sodium agglomerates and untreated Naproxen Sodium drug powder were recorded on FTIR spectro photometer in the wavelength region of 4000-400cm-1. Samples (about 1% w/w) were mixed with KBr powder and suitable amount of sample was placed in a sample holder.
X- ray Diffractometer Study
Powder X-ray diffraction (PXRD) patterns of Naproxen Sodium agglomerates and pure Naproxen Sodium were collected in transmission using an X-ray diffractometer with a rotating anode(Philips, X-pert-MPD) with Cu Kα1 radiation (monochromator: graphite) generated at a 200mA and 40 kV. Powder was packed into the rotating sample holder between two films (PETP). Quantitative determination of crystallinity at same 2θ value suggests the degree crystallinity of drug in formulation relative to degree of crystallinity of pure drug.
RESULTS AND DISCUSSION
Selections of Solvents:
Spherical agglomerates of Naproxen Sodium were prepared by Spherical Agglomeration (SA) using a Methanol. It involves good solvent, poor solvent and a bridging liquid. The selection of these solvents depends on the miscibility of the solvents and the solubility of drug in individual solvent. Accordingly Methanol, dichloromethane, water were selected as a good solvent, bridging liquid, and poor solvent, respectively. Naproxen Sodium is highly soluble in methanol, but poorly soluble in water. Also it is soluble in dichloromethane which is immiscible in water. Hence, this solvent system was used in the present study. In Spherical Agglomeration (SA) method, when good solvent solution of drug plus bridging liquid were poured in the poor solvent under agitation. The counter-diffusion of the poor solvent into the droplets induces the crystallization of the drug within the droplet due to the decrease in solubility of the drug in the droplet containing the poor solvent. The bridging liquid wets the crystal surface to cause binding and promotes the formation of liquid bridges between the drug crystals to form spherical agglomerates. The spherically agglomerated crystals are formed by coalescence of these dispersed crystals. In the present study effect of different polymer on solubility and dissolution rate of spherical agglomerates of Naproxen Sodium were studied.
Effect of Drug: Polymer Ratio
The presence of polymers in spherical agglomerates influenced the particles size of resultant agglomerates. As the concentration of the polymers increased, the size of the agglomerates increased. The presence of polymers on the particle surface increases particle-particle interaction, causing faster squeezing out of DMF to the surface resulting in increased particle size. Table 2 represents the microscopic observation of pure Naproxen Sodium and prepared agglomerated crystals with polymers PVP K30, polyethylene glycol (PEG 400). The agglomerated crystals prepared by incorporating water-soluble polymers can improve solubility and in vitro drug release. Addition of PEG400 water soluble polymer increased the solubility of Naproxen Sodium in water as well as in the dissolution medium. In order to impart strength and sphericity besides increasing the solubility and drug release from agglomerates PVP K30 was selected. As the concentration of the PVP K30 increased, the size of the agglomerates increased.
Table 2: Results of Evaluation Parameters for Agglomerates of Naproxen Sodium
|
Batch |
Angle of |
% Compre |
Particle |
% Yield |
Solubility |
% Drug |
|
Pure |
38.39±0.02 |
12.10±0.05 |
155.62±0.05 |
- |
0.0159±0.051 |
99.98±0.048 |
|
F1 |
32.13±0.026 |
20.81±0.051 |
105.85±0.05 |
98.35±0.034 |
0.351±0.025 |
99.95±0.065 |
|
F2 |
29.35±0.069 |
19.55±0.075 |
128.96±0.02 |
90.81±0.036 |
0.369±0.026 |
98.36±0.042 |
|
F3 |
30.18±0.015 |
19.32±0.048 |
110.26±0.04 |
95.81±0.029 |
0.389±0.065 |
95.36±0.075 |
|
F4 |
28.50±0.025 |
21.85±0.048 |
125.68±0.03 |
90.12±0.056 |
0.356±0.035 |
97.36±0.025 |
|
F5 |
23.28±0.042 |
25.10±0.042 |
92.36±0.05 |
98.75±0.015 |
0.483±0.040 |
99.97±0.042 |
|
F6 |
27.15±0.015 |
23.62±0.042 |
109.35±0.05 |
92.15±0.011 |
0.395±0.023 |
95.69±0.038 |
|
F7 |
28.05±0.052 |
22.81±0.015 |
117.35±0.05 |
93.63±0.0522 |
0.321±0.032 |
98.93±0.026 |
|
F8 |
24.10±0.025 |
15.16±0.0531 |
102.36±0.04 |
96.51±0.023 |
0.324±0.045 |
99.36±0.022 |
|
F9 |
28.50±0.023 |
18.91±0.033 |
115.68±0.02 |
95.14±0.031 |
0.367±0.025 |
99.99±0.012 |
Angle of Repose
Angle of repose was shown in Table 5.2.It was found that angle of repose of the agglomerates decreased as compared to the pure Naproxen Sodium It indicated that there were substantial improvements in flow and of the powder mass in comparison to the drug. Naproxen Sodium had angle of repose 38.39.Angle of repose for batch F1toF9 was found in between 23.28 to 32.13.
% Compressibility
%Compressibility of agglomerates of Naproxen Sodium was between 15.16 to 25.10 as shown in Table 2. % Compressibility of pure Naproxen Sodium was 12.10. Compressibility of agglomerates increased as compare to pure Naproxen Sodium. Result indicated that there was subsequently improvement in %compressibility of agglomerates with addition of polymer like PEG 400 & PVP K-30.
Particle Size
Determination of particle size was done using optical microscopy and results were shown in table 2. Results indicated that particle size range of Naproxen Sodium agglomerates was in between 155.85µm to 185.68µm. Particle size of pure Naproxen Sodium was 155.62 µm. it indicated, there, was satisfactory decreased particle size by preparing agglomerates of drug by incorporating hydrophilic polymers like PEG-4000 and PVP K- 30. Decrease in particle size was improved solubility and dissolution of drug.
% Yield:
The result of % yield was depleted in Table 5.3. The yield of agglomerates was found satisfactory and ranged from 90 to 98 %. Batch F5 showed maximum yield 98.75 %
Solubility Study:
The results of solubility studies indicate that the pure Naproxen Sodium possesses a very low solubility in water was (0.0159±0.0005 mg/ml, n=3). The drug solubility from the spherical crystals increased significantly demonstrating that the incorporation of hydrophilic polymers like PEG 4000 and PVPK30 which enhances the drug solubility by improving wettability. The use of the polymer PVPK30 & PEG400 in agglomerates formation increased the solubility. This may be due to the improved porosity, decreased particle size and partial size and partial amorphization of drug in agglomerates. The solubility also increased due to adsorption of PVPK30 on to the drug particle surface during agglomeration. Addition of polyethylene glycol 400 water-soluble polymer increases the solubility of Naproxen Sodium in water. The solubility of, PEG 4000 and PVP K-30 in distilled water was given Table 2.
Drug content
Result of drug content was showed in table 5.3. All the batches of agglomerates found between 95 to 99 %drug content.
In Vitro Drug Dissolution
In vitro dissolution curve was shown in Figure 1 and Figure 2. It indicated increased drug release than pure Naproxen Sodium.Batch F1 to F5 showed almost complete drug release in 90 to 60min respectively. Among this batch F5 showed total drug release in 60 min. Batch F3 and F4 showed complete drug release in 70 min. Increased drug release might be due to lower particle size and increased amount of PEG 400 in agglomerates. Higher the amount of PEG 400 showed increased dissolution of Naproxen Sodium agglomerates. Batch F6 to F9 showed almost complete drug release in 90 to 70 mins respectively. Among this Batch F-9 to showed complete drug release in 70 mins. Batch F7 and F8 showed drug release in 80 min. increased dissolution of Naproxen Sodium agglomerates may be due to hydrophilic nature of PVPK-30 and lower particle size. Amount of PVPK-30 increased dissolution rate also increased. It may be due to absorption of drug particles on the surface of PVP K -30 during preparationof SA. From all nine formulations batch F5 to F9 showed higher dissolution rate of Naproxen Sodium than pure Naproxen Sodium.
Batch F5 showed complete drug release in 60 min while batch F9 showed complete drug release in 70 min. this may be due to more hydrophilic nature of PEG 400 and lower particle size of agglomerates by incorporation of PEG 400. In case of PVP K -30 particle size was higher due to somewhat more agglomeration of particles.
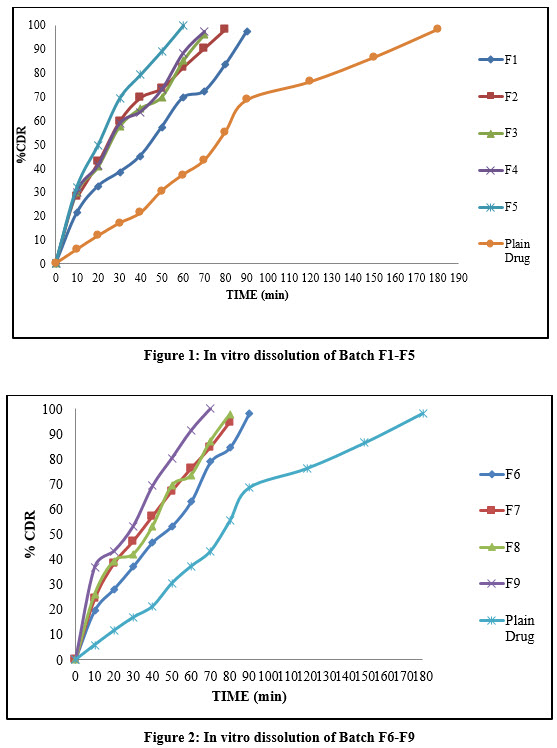
Figure 1: In vitro dissolution of Batch F1-F5 | Figure 2: In vitro dissolution of Batch F6-F9
Physical Characterization of agglomerates of Naproxen Sodium
Differential Scanning Calorimetry Study
DSC thermograms of pure Naproxen Sodium and Naproxen Sodium agglomerates were illustrated in Figure 3.The DSC thermogram showed a sharp endothermic peak for all the Naproxen Sodium crystals The DSC pattern of pure Naproxen Sodium and agglomerates showed a sharp endothermic peak at 158 ºC corresponding to its melting point.
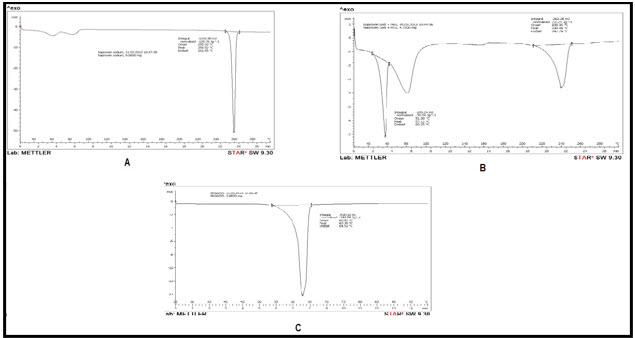
Figure 3: DSC Thermogram of (A) Naproxen Sodium (B) PEG 400 and (C) Agglomerates of Batch F5
X-ray Diffraction Studies
X-ray diffraction patterns of Naproxen Sodium and Naproxen Sodium agglomerates were shown in Figure 4. XRD pattern in 2θ range showed the diffraction peaks, characteristic of Naproxen Sodium. This indicates conversation of crystallinity nature of pure drug into somewhat amorphous nature.
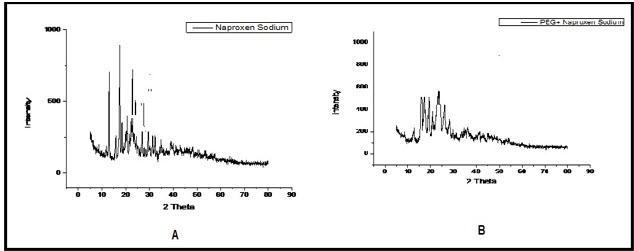
Figure 4: X-Ray Diffractograms of (A) Naproxen Sodium and (B) Batch F5
Fourier Transform Infrared Spectroscopy Studies
FT-IR Spectra of Naproxen Sodium, PEG 400, PVP K-30 and agglomerates was shown in Figure 5. It indicated there was presence of characteristic peak of Naproxen Sodium in a spectrum of agglomerates. Hence, there was no chemical interaction of excipients during preparation of agglomerates and there was no change in chemical structure of drug.
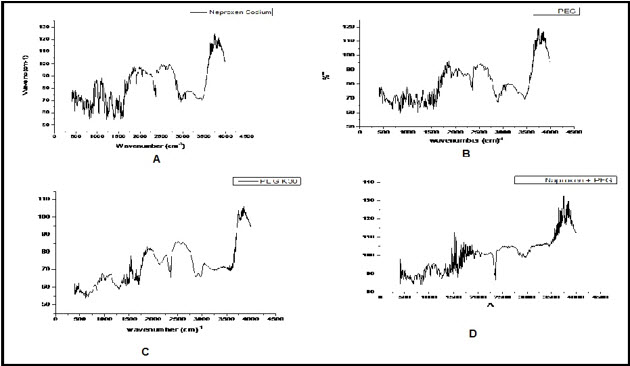
Figure 5: FTIR Spectra of (A) Naproxen Sodium (B) PEG 400 (C) PVP K-30 and (D) Batch F5
CONCLUSION
The manufacturing of spherical crystals by Spherical crystallization technique required simple and common equipment only and is able to perform in a signal step processes. Naproxen Sodium practically insoluble in water and its dissolution is rate limiting step for absorption from GI track, which lead variable bioavailability. Thus, by preparing spherical agglomerates of Naproxen Sodium by Spherical crystallization technique leads to improvement in solubility and dissolution rate of Naproxen Sodium. The aforementioned technique is promising tool for effective agglomerate formulation during pharmaceutical development in order to achieve appropriate dissolution of poorly water soluble ingredient. This technique may be applicable for producing oral solid dosage forms of poorly soluble drugs with improved dissolution rate and solubility.
REFERENCES
1.Franchini MK. Particle Engineering, in Encyclopedia of Pharmaceutical Technology (Ed. J. Swarbrick),3rd ed., Taylor & Francis, New York 2006.
2.Chaumeil JC, Micronization: A method of improving the bioavailability of poorly soluble drugs. Exp. Clin. Pharmacol. 20:211–215, 1998.
3.Feeley JC, York P, Sumby BS and Dicks H. Determination of surface properties and flow characteristics of salbutamol sulphate, before and after micronization, Int. J. Pharm. 172:89–96, 1998.
4.De Villiers MM. Influence of cohesive properties of micronized drug powders on particle size analysis, J. Pharm. Biom. Anal. 13:191–198, 1995.
5.Tiwary AK. Crystal Habit Changes and Dosage Form Performance, in Encyclopedia of Pharmaceutical Technology, 3rd ed., Taylor & Francis, New York, 820–833, 2006.
6.Kaul D, Nguyen NT and Venkataram S. Crystal habit modifications and altered tableting characteristics. Int. J. Pharm. 88:345–350, 1992.
7.Garekani HA, Sadeghi Badiee FA, Mostafa SA and Rajabi-Siahboomi AR. Crystal habit modifications of ibuprofen and their physicochemical characteristics. Drug Dev. Ind. Pharm. 27:803–809, 2001.
8.N Rasenack and Müller BW. Ibuprofen crystals with optimized properties. Int. J. Pharm. 245: 9–24, 2002.
9.Morris KR, Griesser UJ, Eckhardt CJ and Stowell JG. Theoretical approaches to physicaltransformations of active pharmaceutical ingredients during manufacturing processes. Adv. DrugDel. Rev. 48: 91–114, 2001.
10.Kawashima Y and Capes CE. An experimental study of the kinetics of spherical agglomeration in a stirred vessel. Powder Technol. 10:85–92, 1974.
11.Kawashima Y. Development of spherical crystallization technique and its application to pharmaceutical systems. Arch. Pharm. Res. 7:145–151, 1984.
12.Rodriguez-Hornedo N, Kelly RC, Sinclair BD and Miller JM. Crystallization: General Principles and Significance on Product Development. Encyclopedia of Pharmaceutical Technology (Ed. J.Swarbrick), 3rd ed., Taylor & Francis Group, New York, 834–857, 2006.
13.Myerson AS and Ginde R. Crystals, Crystal Growth, and Nucleation, in Handbook of IndustrialCrystallization (Ed. A. S. Myerson), 2nd ed., Butterworth-Heinemann, Woburn, 33–65, 2001.
14.Mullin JW. Crystal Growth, in Crystallization (Ed. J. W. Mullin), 4th ed., Butterworth-Heinemann, Woburn, 216–288, 2001.
15.Sekerka RF. Theory of Crystal Growth Morphology, in Crystal Growth: From Fundamentals to Technology (Eds. G. Müller, J. Métois and P. Rudolph), Elsevier, Amsterdam , 55–93, 2004.
16.Kawashima Y, Okumura M and Takenaka H. The effects of temperature on the spherical crystallizationof salicylic acid. Powder Technol. 39:41–47,1984.
17.Göczo H, Szabó-Révész P, Farkas B, Hasznos-Nezdei M, Serwanis SF, Pintye-Hódi AK, Kása P, Eros JrI, Antal I and Marto S. Development of spherical crystals of acetylsalicylic acid for direct tablet-making. Chem. Pharm. Bull. 48:1877–1881, 2000.
18.Blandin AF, Mangin D, Rivoire A, Klein JP and Bossoutrot JM. Agglomeration in suspensionof salicylic acid fine particles: influences of some process parameters on kinetics and agglomerate final size. Powder Technol. 130:316–323, 2003.
19.Kawashima Y, Furukawa K and Takenaka H. The physicochemical parameters determining the size f agglomerate prepared by the wet spherical agglomeration technique. Powder Technol. 30:211–216, 1981.
20.Bos AS and Zuiderweg FJ. Kinetics of continuous agglomeration in suspension. Powder Technol. 44 (1985) 43–51.
21.Sadowski Z. Selective spherical agglomeration of fine salt-type mineral particles in aqueous solution. Colloids and Surf. A: Physicochem. Eng. Aspects 96:277–285, 1995.
22.Skarvelakis C and Antonini G. Kinetics of agglomerate growth in a continuous coal-oil purification process. Powder Technol. 85:135–141, 1995.
23.Snyder BA and Berg JC. Oil-assisted agglomeration for toner deinking: Population balancemodel and experiments. AlChE J. 43:1480–1487, 1997.
24.Laskowski JS and Yu Z. Oil agglomeration and its effect on beneficiation and filtration of lowrank/oxidized coals. Int. J. Min. Proc. 58:237–252, 2000.
25.House CI and Veal CJ. Selective recovery of chalcopyrite by spherical agglomeration. Min. Eng. 2:171–184, 1989.
26.Huang AY and Berg JC. Gelation of liquid bridges in spherical agglomeration. Colloids andSurf. A: Physicochem. Eng. Aspects 215:241–252, 2003.
27.Mahanty S, Sruti J, NiranjanPatra C and BhanojiRao ME. Particle design of drugs by spherical crystallization techniques. Int. J. Pharm. Sci. Nanotech. 3:912–918, 2010.
28.Thati J and Rasmuson ÅC. On the mechanisms of formation of spherical agglomerates. Eur. J. Pharm. Sci. 42:365–379, 2011.
29.Kawashima Y, Cui F, Takeuchi H, Niwa T, Hino T and Kiuchi K. Parameters determining the agglomeration behaviour and the micromeritic properties of spherically agglomerated crystals prepared by the spherical crystallization technique with miscible solvent systems. Int. J. Pharm.119:139–147, 1995.
30.Katta J and Rasmuson AC. Spherical crystallization of benzoic acid. Int. J. Pharm. 348:61–69, 2008.
31.Zhang H, Chen Y, Wang J and Gong J. Investigation on the spherical crystallization process of cefotaxime sodium. Ind. Eng. Chem. Res. 49:1402–1411, 2010.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









