About Authors:
Roohi Kesharwani1*, Devendra Singh2, Vishal Jacob1
1-Institute Of Foreign Trade And Management Lodhipur Rajput,
Delhi Road, Moradabad, India
2-Raj Kumar Goel Institute Of Technology, Delhi-Meerut Road, Ghaziabad, India
*roohi4mail@gmail.com, devendrasingh.pisces@gmail.com
ABSTRACT:
Pharmacovigilance is now accepted to be a continuous process of evaluation accompanied by steps to improve safe use of medicines which involves pharmaceutical companies, regulatory authorities, health professionals and patients. The methodologies have broadened to encompass many different types of study, with spontaneous reporting remaining the cornerstone. The concern for ADRs in highly vulnerable populations is of even greater concern.Pharmacovigilance is especially important since most of the adverse effects are reversible by modifying the dosage or omitting the offending medicine. All medicines (pharmaceuticals and vaccines) haveside effects.In a vast country like India with a population of over 1.2 Billion with vast ethnic variability, different disease prevalence patterns, practice of different systems of medicines, different socioeconomic status, it is important to have a standardized and robust pharmacovigilance and drug safety monitoring programme for the nation. Collecting this information in a systematic manner and analyzing the data to reach a meaningful conclusion on the continued use of these medicines is the rationale to institute this program for India. In this review article various hospitals survey are done and the survey questionnaire was analyzed question wise and their percentage value was calculated. Our study strongly suggests that there is greater need to create awareness and to promote the reporting of ADR among healthcare professionals of the country. Only such approach can greatly influence in bringing reporting culture among healthcare professionals and may improve the reporting rates of ADR in our country. Pharmacists, as doctors opined that their involvement may increase the reporting rate, have a greater role to play in the area of pharmacovigilance.
REFERENCE ID: PHARMATUTOR-ART-1676
INTRODUCTION:
Pharmacovigilance: The is defined by WHO as “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible drug?related problems”. [1]
Drug toxicity is a relatively common phenomenon—despite a stringent drug safety and clinical trials process, several drugs have been removed from the market being approved by National Drug Regulatory Authorities, including US’ FDA, UK’s MHRA, and Europe’s EMEA [refer to annex of drug withdrawals]. In addition to the removal of potentially toxic drugs from the market, one out of every five drugs[1] are required to add additional warnings related to side?effects, contraindications, etc. Globally, only about 500,000 to 700,000 adverse event occurrences are captured each year[2]—however, low?to middle?income countries, which represent more than two?thirds of the world’s population account for a tiny fraction of all the ADR data.
The concern for ADRs in highly vulnerable populations is of even greater concern. For example, in pediatrics, antiretroviral treatment (ART) intolerance and toxicity is a major cause of poor adherence, changing medications and eventually dropping out of a treatment program. “Adverse effects associated with antiretroviral medicines have been reported to occur in up to 30% of HIV?infected children on antiretroviral therapy.” Pharmacovigilance is especially important since “most of the adverse effects are reversible by modifying the dosage or omitting the offending medicine.”[1, 2, 3, 4]
The Pharmaceutical industry in India is valued at Rs. 90,000 Crore and is growing at the rate of 12 – 14 % per annum. Exports are growing at 25 % Compound Annual Growth Rate (CAGR) every year. The total export of Pharma products is to the extent of Rs. 40,000 Crore. India is now being recognized as the ‘Global pharmacy of Generic Drugs’ & has distinction of providing generic quality drugs at affordable cost. India is also emerging rapidly as a hub of Global Clinical trials & a destination for Drug Discovery & Development.
This is reflected in the fact that total number of applications received & processed has more than doubled from around 10,000 in the Year 2005 to 22,806 in Year 2009 at CDSCO, HQ, New Delhi. This includes increase in New Drug Applications, Global Clinical Trials , Market Authorization of Vaccine & Biotech products from 1200 ,100 ,10 in Year 2005 to 1753, 262 & 137 in the Year 2009 respectively.
All medicines (pharmaceuticals and vaccines) haveside effects.In a vast country like India with a population of over 1.2 Billion with vast ethnic variability, different disease prevalence patterns, practice of different systems of medicines, different socioeconomic status, it is important to have a standardized and robust pharmacovigilance and drug safety monitoring programme for the nation. Collecting this information in a systematic manner and analyzing the data to reach a meaningful conclusion on the continued use of these medicines is the rationale to institute this program for India. Since, there are considerable social and economic consequences of ADRs there is a need to engage health-care professionals, in a well structured programme to build synergies for monitoring ADRs. The purpose of the Pharmacovigilance Program of India is to collect, collate and analyze data to arrive at an inference to recommend regulatory interventions, besides communicating risks to healthcare professionals and the public.[5]
FUTURE PROPECTS:
Pharmacovigilance has been expanding in recent years, as companies are required to monitor drug safety post launch. Drug safety issues, such as those raised by Vioxx earlier this decade, have led to increased risk-averseness by regulators, with greater post-marketing assessment of drugs. Many regulatory agencies require detailed pharmacovigilance, with companies bearing extra costs, our new report also observes. Healthcare payers, prescribers and patients have high expectations from pharmacovigilance. They want thorough information - on adverse reactions and overall drug safety - upon which to make informed judgements.
Pharmacovigilance is now being called upon to produce clear results, expressed openly. What will those trends mean for pharmacovigilance, from the perspectives of major stakeholders, including the pharma and biotech industries? Where is pharmacovigilance heading? What regulatory measures will continue, and which new processes will emerge? This new report - Pharmacovigilance 2009: Present Challenges and Future Goals- explains how that field will develop from the present onwards.
Clearly, pharmacovigilance is increasingly important worldwide, especially to avoid reoccurrences of serious, costly problems damaging to the industry. Pharmacovigilance is designed to provide crucial data on how drugs work in medical practice, from the short-term to the long-term. This information can aid drug development and marketing if harnessed properly, being a boon rather than a hindrance. In particular, visiongain believes that live licensing will form a significant part of pharma regulations and drug development in coming years. Pharmacovigilance will underpin processes and developments such as these, as this report further explains.[6]
Medicines have helped to bring improved health and longer life to human beings. Medicines affect the lives of hundreds of millions of people every day. But they are not without risk, and have caused, do cause and will continue to cause harm to many people. There are also large numbers of people who experience no evident effect at all from the drugs they take.[5,6]To be eternally vigilant to ensure that medicines, which are developed for treatment of diseases, actually do not do more harm than good, is one of the important pre-requisites for the progress of medicine.
PLAN OF STUDY
· Study of steps involved in ADR reporting.
· Collection of data of pharmacovigilance andits status in India.
· Pharmacovigilance status in respect to Ayurvedic/ herbal medicine.
· Survey on ADR reporting awareness of physicians at surrounding hospitals.
· Importance of Pharmacy profession in ADR reporting.
LITERATURE REVIEW
Pharmacovigilance is characterized by the fact that it derives its knowledge about the safety of drugs from the clinical usage of drugs in daily practice. Pharmacovigilance is a two-way system, which is represented by the circle of knowledge and practice. [7, 8]
In the literature pharmacovigilance is frequently put on a par with Post Marketing Surveillance. This approach highlights pharmacovigilance’s most visible method, viz. the spontaneous reporting system (SRS).[9]
Flow chart of the flow of information/ different level of centre involved:
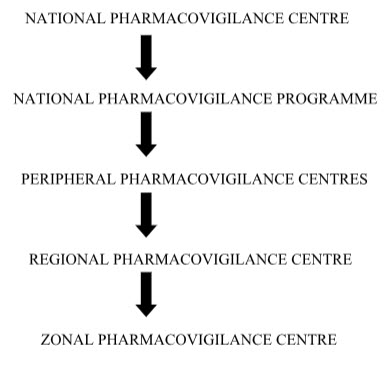
NATIONAL PHARMACOVIGILANCE CENTRE:
The Central Drugs Standard Control Organization (CDSCO) has initiated a country-wide Pharmacovigilance programme under the aegis of DGHS, Ministry of Health & Family Welfare Government of India.
The programme is coordinated by the National Pharmacovigilance Centre at CDSCO. The National Centre is operating under the supervision of the National Pharmacovigilance Advisory Committee to recommend procedures and guidelines for regulatory interventions.
National pharmacovigilance’s programme:
The National Pharmacovigilance Programme was officially inaugurated by the Honorable Health Minister Dr.Anbumani Ramadoss on 23 November, 2004 at New Delhi. The National Pharmacovigilance Programme for India, sponsored by the World Health Organization (WHO) and funded by the World Bank, became fully operational in January 2005.
The Programme aims to foster the culture of ADR notification in its first year of operation and subsequently aims to generate broad based ADR data on the Indian population and share the information with global health-care community through WHO-UMC.The nationwide programme, sponsored and coordinated by the country’s central drug regulatory agency – Central Drugs Standard Control Organization (CDSCO) – to establish and manage a data base of Adverse Drug Reactions (ADR) for making informed regulatory decisions regarding marketing authorization of drugs in India for ensuring safety of drugs.[7]
Under the program 26 peripheral centers, 5 Regional Centers and 2 Zonal Centers were established. The Peripheral centers will record the Adverse Events (AE) and send to the Regional Centers.They in turn collate and scrutinize the data received from the Peripheral Centers and submit to the Zonal Centers. The Zonal Centers will analyze the data and submit consolidated information to the National Pharmacovigilance Centre. The Zonal Centre will also provide training, general support and coordinate the functioning of the Regional Center
Peripheral pharmacovigilance centers:
Primary pharmacovigilance’s centers. Relatively smaller medical institutions including individual medical practitioners’ clinics, private hospitals, nursing homes, pharmacies etc. First contact ADR data collection unit at a health care facility. They would be identified and coordinated by RPCs / ZPCs in consultation with CDSCO.
Regional Pharmacovigilance Centers (RPCs):
Secondary pharmacovigilance centers. Relatively larger healthcare facilities attached with medical colleges. They would act as second level centers in the administrative structure of the nppi. They will function as first contact adr data collection units also. They would be identified and coordinated by zpcs in consultation with the cdsco.
Zonal Pharmacovigilance:The Centre (ZPCs) :
Tertiary pharmacovigilance centers. Large healthcare facilities attached with medical colleges in metro cities identified by the CDSCO for the purpose. They would act as third level centers inthe administrative structure of the NPPI. They will function as First contact ADE data collection units also.[7]
THE NATIONAL PHARMACOVIGILANCE ADVISORY COMMITTEE (NPAC):
Oversee the performance of various Zonal, Regional and Peripheral Pharmacovigilance centers as well as recommend possible regulatory measures based on the data received from various centers. It also oversees data collection and assessment, interpretation of data as well as publication of ADR monitoring data. The Committee also periodically evaluates their protocol compliance levels to ensure that the data received is homogenous and can be scientifically pooled for informed regulatory decisions. Wherever necessary, NPAC also seeks the opinion of experts in various specializations.
The specific aims of the Pharmacovigilance Programme are to:
· Contribute to the regulatory assessment of benefit, harm, effectiveness and risk of medicines, encouraging their safe, rational and more effective (including cost effective) use.
· Improve patient care and safety in relation to use of medicines and all medical and paramedical interventions.
· Improve public health and safety in relation to use of medicines.
· Promote understanding, education and clinical training in pharmacovigilance and its effective communication to the public.
· Monitoring medicines as used in everyday practice to identify previously
· unrecognized adverse effects or changes in the patterns of their adverse effects
· Assessing the risks and benefits of medicines in order to determine what action,
· if any, is necessary to improve their safe use
· Providing information to users to optimize safe and effective use of medicines
· Monitoring the impact of any action taken [10]
ADR REPORTING:
An adverse drug reaction (abbreviated ADR) is a term used to describe the unwanted, negative Consequences sometimes associated with the use of medications. ADR is a particular type of Adverse effect.[11]
“Adverse drug reaction" or an "adverse reaction" means a response to a medicine in the humans or animals, which is noxious and unintended, including lack of efficacy, and which occurs at any dosage and can also result from an overdose, misuse or abuse of a medicine.[12]
Figure1: ADR (ADVERSE DRUG REACTION ) ANALYSIS CHART
The need for an effective risk management strategy was recognized by FDA as early as 1993 when Med Watch, the safety information and adverse event reporting program of FDA was established. Currently the evaluation and risk assessment of drugsis taken care by a separate division of FDA, the Pharmacovigilance and Epidemiology Division of CDER (Center for Drug Evaluation and Research).[13]
FDA (1996), stated that the success or failure of any pharmacovigilance activity dependson the reporting of suspected adverse reactions.[14]
The objectives of the ADR reporting system are to receive adverse events suspected to be related to the use of medication, to evaluate drug information from reported cases as well as from published literatures, to collect drug safety information internationally, to created information feedback mechanisms by publishing drug safety newsletters, and to carry out education program. The ultimate goal is to enhance rational drug usage and hence to improve public health, (DOH 1998).[15]
According to WHO (2002), reporting of ADRs can be done by Doctors, Nurses, Pharmacists and any other health worker. The statement said that the reports can be sent to the Pharmacy Department, Hospital or the National Pharmacovigilance centre in each country. While making report on ADRs it is also important to include; Patient details (initials)Suspected drug name, strength, dose and duration of treatment. The report should also include information on other concomitant drugs, Type of ADRs, i.e All ADRs, New ADRs, ADRs in risk groups like pregnant women, breastfeeding women, elderly, children etc. Drug interactions, Serious/unexpected ADRs, Other drug related problems due to quality, inappropriate use etc is considered important as well.[16]
STEPS INVOLVED IN STUDY:
Adverse drug reactions can occur when the body's immune system reacts with the chemical compound in a drug, report doctors at the American Academy of Family Physicians. Other reactions happen as result of allergies. Unknown causes of adverse drug reactions can happen when a diagnosis is not clear or a patient's medical history is in question. While treatment usually involves discontinuing the offending drug, you need to know the best way report adverse drug reactions to receive the proper treatment.
Step 1
Keep the prescription bottles of your medications so healthcare providers can get the exact name of the drug that you are taking when you have a reaction. AAFP doctors report that most drug reactions manifest as a rash, but can be severe and cause unconsciousness.
Step 2
Be prepared to report any allergic reactions you may have had in the past to help doctors diagnose your symptoms. Keep a record of when you started taking a new medication. Have available a list of all medications you are currently taking, including the dosage amount.
Step 3
Show treating physicians any skin rash or other skin abnormality. You may have a fever and trouble breathing, but any number of factors could account for those symptoms. When combined with a skin discoloration or lesion, an adverse drug reaction diagnosis is easier to make.
Step 4
Document the treatment you received that caused the reaction as well as the results. Keep track of dates, medical complications you underwent, the level of your reaction and how you were treated so you can follow up with the proper reporting procedures if your doctor refuses to report your reaction.
Step 5
Print the Food and Drug Administration reporting form from the FDA website and bring it to your doctor to fill out. If you prefer not to go through your healthcare provider, the FDA does accept reports of adverse drug reaction from consumers. This is a voluntary program that is monitoredby the FDA to follow patients' experiences with various medications.
Step 6
Call the FDA at (800) FDA-0178 if you do not have access to online reporting. Use the FDA to report fraud or misuse of drugs as well as adverse reactions to prescribed medication, medical devices or over-the-counter medications or supplements.[17]
COLLECTION OF DATA OF PHARMACOVIGILANCE AND ITS STATUS IN INDIA:
The National Pharmacovigilance Advisory Committee (NPAC) monitors the performance of various zonal, regional, and peripheral centers and performs the functions of "Review Committee" for this program. The NPAC also recommends possible regulatory measures based on pharmacovigilance data received from various centers. The Zonal Pharmacovigilance Centre (ZPC) and Regional Pharmacovigilance Centre (RPC) have also been established.
The Central Drugs Standard Control Organization (CDSCO)[3] is initiating a countrywide pharmacovigilance program under the aegis of DGHS, MoH and Family Welfare, and Government of India. The National Pharmacovigilance Centre at CDSCO shall coordinate the program. The National Centre will operate under the supervision of the NPAC to recommendpicadors and guidelines for regulatory interventions.
The National Pharmacovigilance Program will have the following milestones:
- Short-term objectives: To foster a culture of notification.
- Medium-term objectives: To engage several healthcare professionals and Non-Government Organizations (NGOs) in the drug monitoring and information dissemination processes.
- Long-term objectives: To achieve such operational efficiencies that would make Indian
- National Pharmacovigilance Program a benchmark for global drug monitoring endeavors.
Periodic Safety Update Reports shall be expected to be submitted every 6 monthly for the first 2 years of marketing in India, and annually for the subsequent 2 years. In addition, training programs and interaction meetings shall be held every 6 months after the initial training.
All data generated (including reporting forms) will be stored and preserved for the purpose of archiving for a minimum period of 5 years at the ZPCs. The reporting of seemingly insignificant or common adverse reactions would be important because it may highlight a widespread prescribing problem.[12,19]
PHARMACOVIGILANCE IN RESPECT TO AYURVEDIC AND HERBAL MEDICINE:
AYURVEDA:
Ayurveda, the knowledge of life, immortalized in the form of elegant Sanskrit stanzas in the samhitas describe diagnosis and therapy of disease as well as ways to maintain positive health.[20,21] Although the technical term "Pharmacovigilance" does not feature in Ayurveda texts, the spirit of pharmacovigilance is vibrant and is emphasized repeatedly in all major texts. The major goals of pharmacovigilance, namely to improve patient care and safety in relation to drug use, and thus promote rational drug use are recurrent themes of ayurvedic pharmacology (dravyaguna vigyan ) and therapeutics (chikitsa ).[22]The use of ayurvedic medicines is popular in India - and in recent times has become accepted in other countries. For example, a recent survey conducted by the NCCAM in the USA showed that about 751 000 people in the United States had ever used ayurveda and 154 000 people had used them within the past 12 months.[23]Associated with this increasing use, are growing concerns about the safety of ayurvedic medicines.[24,25] This paper discusses in brief the ayurvedic concepts of adverse reactions to medicines, the need for pharmacovigilance of ayurvedic medicines, challenges in introducing pharmacovigilance in ayurveda and some recommendations to successfully implementing these activities.
Ayurveda Concepts of Adverse Reactions:
There is a popular misconception that ayurvedic medicines are devoid of adverse reactions. However, the Charaka Samhita , which is a classic text book of ayurveda, describes all the adverse reactions to medicines when they are prepared or used inappropriately. Attention is given to factors like the physical appearance of the part of the plant to be used (prakriti ), its properties (guna ), actions (karma; prabhava ), habitat (desh ), season in which it grows (ritu ), harvesting conditions (grahitam ), method of storage (nihitam ) and pharmaceutical processing (upaskritam ), which must be considered while selecting the starting material that goes to form the medicine.[26] Similarly, Charaka also describes, elegantly, several host-related factors to be considered when selecting medicines in order to minimize adverse reactions like the constitution of the patient (prakriti ), age (vayam ), disease (vikruti ), tolerance (previous exposure) (satmya ), psychological state (satwa ), digestive capacity (ahara-shakti ), capacity for exercise (vyayama shakti ), quality of tissues (Sara ), physical proportions of the body (sahanan ) and strength (bala ).[27]
There is a popular misconception that ayurvedic medicines are devoid of adverse reactions. However, the Charaka Samhita , which is a classic text book of ayurveda, describes all the adverse reactions to medicines when they are prepared or used inappropriately. Attention is given to factors like the physical appearance of the part of the plant to be used (prakriti ), its properties (guna ), actions (karma; prabhava ), habitat (desh ), season in which it grows (ritu ), harvesting conditions (grahitam ), method of storage (nihitam ) and pharmaceutical processing (upaskritam ), which must be considered while selecting the starting material that goes to form the medicine.[26] Similarly, Charaka also describes, elegantly, several host-related factors to be considered when selecting medicines in order to minimize adverse reactions like the constitution of the patient (prakriti ), age (vayam ), disease (vikruti ), tolerance (previous exposure) (satmya ), psychological state (satwa ), digestive capacity (ahara-shakti ), capacity for exercise (vyayama shakti ), quality of tissues (Sara ), physical proportions of the body (sahanan ) and strength (bala ).[27]
Interestingly, classical ayurveda prescribes metals and minerals as medicines given as bhasmas (incinerated mineral formulations) or in combination with plants as herbo-mineral formulations (e.g., Arogyavardhini ). Manufacturing procedures for these medicines are stringent, and adverse reactions are described when precautions are not taken while manufacturing and administering these medicines.[28] Although these medicines are widely used in India, doubts about their long-term safety come up due to the presence of toxic metals in them[29] and there are reports related to adverse reactions.[30]
To summarize, Charaka says, "that even a strong poison can become an excellent medicine if administered properly. On the other hand even the most useful drug can act like a poison if handledcarelessly".[31]
Need for Pharmacovigilance of Ayurvedic Medicines:
Recognized by the Government of India as a formal medical system, institutionalized training in Ayurveda was initiated a century ago and now India has 196 under-graduate colleges and 55 post-graduate centers.[32] The number of practitioners registered with the State Registers of Indian Medicine is approximately 438 721.[33]
In ancient times, the ayurvedic physicians prepared medicines for their patients themselves. Today, only a handful of practitioners follow this practice and production and sale of ayurvedic drugs has become formalized into a thriving industry. Manufacture and marketing of ayurvedic drugs is covered by the Drugs and Cosmetics Act, 1940.[34] Broadly speaking, two categories of medicines labeled as "Ayurveda" are available in the market: firstly, classical ayurvedic formulations, which are as per descriptions in Ayurvedasamhitas (e.g., kutajarishta, chandraprabhavati, etc.) and secondly patent and proprietary formulations made of extracts of herbs.[35] There are 8403 licensed Ayurveda pharmacies and the approximate turnover of this industry is Rs. 4000 crore, which accounts for nearly a third of the total pharmaceutics business in India.[36] This commercialization has brought with it many challenges about safe use of Ayurveda medicines, bringing into focus the need for formal pharmacovigilance programs in the field.
And yet, the number of adverse reactions to Ayurveda drugs reported or recorded in the National Pharmacovigilance Program in India is negligible. The strong belief that Ayurveda medicines are safe contributes to a large extent to this situation. To compound this matter is the lack of knowledge about the concept and importance of pharmacovigilance in Ayurveda among Ayurvedapractitioners.
A recent survey conducted among Ayurveda physicians by our department examined their attitudes toward adverse reactions of Ayurveda medicines and reporting these to authorities. Of the 80 vaidyas interviewed, 14 refused to accept that ayurvedic drugs could produce adverse reactions and the rest felt that adverse reactions would occur only if ayurvedic drugs were improperly manufactured and irrationally prescribed. Of these 66 doctors, 48 physicians said that they had seen "unexpected" reactions after administration of Ayurveda drugs in their practice. Interestingly, only 14 of these 48 said that they had reported these reactions (mostly in medical association meetings or to medical representatives) (personal communication).
Challenges in Introducing Pharmacovigilance in Ayurveda:
Although the National Pharmacovigilance Program has encouraged reporting of all suspected drug-related adverse events including those caused by herbal/traditional/alternative medicines (Protocol of NPP, Version 1, 2004, p. 17), the number of reports related to ayurvedic/herbal drugs has been abysmally low. Several challenges that preclude identification and reporting of adverse reactions to ayurvedic drugs can be identified related to detection, assessment and prevention of adverse reactions.
Detection of adverse reactions to Ayurveda medicines:
Perhaps because of the firm belief among doctors and prescribers alike, that ayurvedic drugs are safe, the detection of adverse reactions to these medicines is a major challenge. From obtaining a correct history, to diagnosis and to pin-pointing the causal medicine, the path is full of obstacles, including:
- The concept and terminologies related to adverse reaction monitoring are not covered in the Ayurveda curriculum precluding accurate identification of adverse reactions.
- Methods to study drug safety problems have not evolved adequately in ayurveda.
- Although information related to medicines exists in the stanzas in the ancient treatises of Ayurveda, it is not easily accessible.
- Signal detection is difficult because there is an inherent belief about safety of ayurvedic medications leading to lack of reporting and collection of reports relating to any formulation.
- Patients often use medicines from different systems of medicine concomitantly leading to difficulties in assigning causality.
- Lack of quality assurance and control in manufacture of ayurvedic medicine, which acts as a confounding factor in diagnosing the adverse reaction.
- The informal sector manufacturing and selling ayurvedic drugs on a small-scale is large and this often makes it impossible to identify the medicine that may be causing the adverse reaction. At our Center, for example, we receive adverse reaction reports or requests for testing medicines for adulteration with steroids. Of the total 154 requests of adverse reactions to ayurvedic medicines we have received over the past 5 years, we know the exact ingredients (because of labeling) of ONLY 22 formulations! 132 were from the informal sector - dispensed by the "doctor".
- The problem of counterfeit and spurious drugs is serious. A disturbing trend noticed at our Center is that of masquerading orthodox modern medicines as "Ayurvedic" drugs. Three patients referred to the anti-epileptic clinical pharmacology out-patients department gave a history of receiving "Ayurvedic" medicine for the treatment of epilepsy. They were complaining of giddiness or gingival hyperplasia - both adverse effects associated with anti-epileptic medicines. We found their plasma had carbamazepine and phenytoin in the toxic ranges, and the capsules they were taking, which were analyzed, had 30 and 100 mg of carbamazepine and phenytoin respectively![37]
Assessment of adverse reactions to ayurvedic medicines:
Although several scales are available for causality assessment, applying them for ayurvedic medicines and ascribing causality is perhaps the greatest challenge for several reasons, including:
- Information related to adverse effects is scattered in ayurvedic literature and not in electronic form, hence making it is difficult to access. Many publications are not in peer-reviewed journals and the quality of available publications is questionable.
- Most ayurvedic formulations are multi-ingredient-fixed dose formulations rarely prescribed alone (i.e., there are multiple herbal and herbo-mineral FDCs being consumed at the same time).
- Additionally, there is the confounding factor that the patient is often receiving allopathic medicines at the same time.
- Pharmacokinetics and toxicokinetics are very difficult, and at this point of time, well nigh impossible making definite causality virtually impossible.
- Dose-related responses are rarely measured and reported.
- Rarely, if ever, is de-challenge and re-challenge performed and there is no objective evidence of the adverse event.
- One of the most challenging aspects is the lack of expertise in performing causality analysis with ayurvedic medicines. A person trained in pharmacovigilance rarely understands ayurveda while an expert in ayurveda is not trained in the science of pharmacovigilance.
Prevention of adverse reactions to ayurvedic medicines:
The success in any pharmacovigilance system is in the ability to prevent further adverse reactions successfully by understanding and using the information collected. With ayurvedic medicines, the challenges would be at multiple levels.
- Communication between the practitioners and policy makers of orthodox Western medicine and traditional Indian medicine is not adequate. In India, the current NPVP does not have ayurveda under its fold and therefore ayurvedic practitioners are not aware of the need to report and where to report.
- Unbiased drug information about ayurvedic drugs including both classical and proprietary formulations is not available easily.
- Patients are not adequately aware that ayurvedic medicines can cause adverse reactions and can take medicines for years on end with no monitoring as they believe that these medicines can do no harm. Hence, they do not even give history of taking these medicines.
- Education in ayurveda or modern medicine at both under-graduate and post-graduate levels does not cover pharmacovigilance of ayurvedic medicines, thus never exposing the young physicians to this concept.
- The ayurvedic pharmaceutical industry is not motivated to focus on pharmacovigilance of ayurvedic medicines. Hence, there is no attempt at generating safety data - either before or after marketing of the formulation.
- Availability of ayurvedic medicines is unprecedented in India! It is reported that there are over 100 books describing different ayurvedic medicines containing over 100 000 recipes for medicines![38] The formal ayurvedic formulary quotes over 630 formulations in its two published volumes. Add to that the huge informal sector, the numbers are mind boggling. Which medicines to include in the pharmacovigilance system?
Recommendations:
Based on these observations, there are several ways we can move forward in attempting to embrace pharmacovigilance systems into ayurveda.
- Introduce pharmacovigilance concepts into the curriculum of ayurveda at the under-graduate and post-graduate level.
- Encourage studies on drug safety.
- Make reporting of adverse reactions to regulators mandatory for ayurvedic formulations.
- Make unbiased and easily accessible drug information available. The Traditional Knowledge Digital Library launched by the Government of India[39] is an example of how ancient knowledge available in the ancient scriptures can be made digitally accessible.
- Create awareness about the science of pharmacovigilance among ayurvedic physicians, patients and paramedical staff.
- Development and validation of scales to assess the causality of the reported reactions to ayurvedic medicines.
- Human resource development is a key feature for the success of this enterprise. It will be necessary to train ayurvedic experts in the science of Pharmacovigilance and include them not only in reporting but also assessment of the adverse reactions. More direct involvement of ayurvedic Academic Institutions in the NPVP after appropriate training would be an appropriate first step in this direction. A strong cooperative effort from experts in Pharmacovigilance and ayurveda together can ensure that this system is up and functioning.
Pharmacovigilance in ayurvedic medicines is perhaps an unthought-of concept as yet; however, we do not need an "Ayurvedic thalidomide" to wake the pharmacovigilance community to the need of the hour.
HERBAL:
Pharmacovigilance of Herbal Medicines
The safety of herbal medicines has become a major concern to both national health authorities and the general public[41]. The use of herbs in Traditional medicines continues to expand rapidly across the world. Many people now take herbal medicines or herbal products for their health care in different national health-care settings. However, mass media reports of adverse events tend to be sensational and give a negative impression regarding the use of Herbal medicines in general rather than identifying the causes of these events, which may relate to a variety of issues.[42]
The use of herbal and traditional medicines raises concerns in relation to their safety. [43, 44] There is wide misconception that ‘natural’ means ‘safe’. There is the common belief that long use of a medicine, based on tradition, assures both its efficacy and safety. There are examples of traditional and herbal medicines being adulterated or contaminated with allopathic medicines, chemicals such as corticosteroids, non-steroidal anti-inflammatory agents and heavy metals. Many traditional medicines are manufactured for global use and they have moved beyond the traditional and cultural framework for which they were originally intended. Self-medication further aggravates the risk to patients. When traditional and herbal medicines are used in conjunction with other medicines there is the potential of serious adverse drug interactions.
As with other products intended for human use (medicines, dietary supplements and foods), herbal medicines should be incorporated within a regulatory framework. These products should be governed by standards of safety, quality and efficacy that are equivalent to those required for other pharmaceutical products. Difficulties in achieving this arise from the growth of an ambiguous zone between foods and medicines, into which an increasing number of herbal products fall. The regulatory status of herbal products differs significantly from country to country. Currently less than 70 countries regulate herbal medicines and few countries have systems in place for the regulation of traditional health practitioners.
These disparities in regulation between countries have serious implications for international access to and distribution of such products. For instance, in one country a herbal product may be obtainable only on prescription and from an authorized pharmacy, whereas in another country, it may be obtainable from a health food shop, or even, as has become common practice, by mail order or Internet.
For all these reasons, inclusion of herbal and traditional medicines in national pharmacovigilance programmers has become important and inevitable. Healthcare providers, including traditional health practitioners, regulators, manufacturers and the public share a responsibility for their informed and safe use. The World Health Organization has produced guidelines for assessment of the safety, efficacy and quality of herbal medicines.[45]
New systematic approaches for monitoring the safety of plant-derived medicinal products are being developed.[46] A number of national pharmacovigilance centres are now monitoring the safety of traditional medicines. For that to succeed, the collaboration and support of consumers, traditional health practitioners, providers of traditional and herbal medicines and other experts is necessary. More attention needs to be given to research and to training of healthcare providers and consumers inthis area.
Herbal medicines consist of plant or its part to treat injuries, disease or illnesses and are used to prevent and treat diseases and ailments or to promote health and healing. It is a drug or preparation made from a plant or plants and used for any of such purposes. Herbal medicines are the oldest form of health care known to mankind .[46,47,48] World Health Organization (WHO) has defined herbal medicines as finished, labeled medicinal products that contain active ingredients, aerial or underground parts of the plant or other plant material or combinations. World Health Organization has set specific guidelines for the assessment of the safety, efficacy, and quality of herbal medicines. WHO estimates that approx 81% of the world populations presently use herbal medicine for primary health care.[49]
Adverse Drug Reactions: Herbal remedies are not entirely free of adverse drug reactions. Some adverse drug reactions of commonly used herbs are, Ginkgo biloba cause spontaneous bleeding, St. John’s Wort(Hypericum perforatum) cause gastrointestinal disturbances, allergic reactions, fatigue, dizziness, photosensitivity, confusion, Capsicum annuum cause hypertension, cardiac arrhythmias, myocardial infarction, Ephedra cause anxiety, Vitex agnus (Chast tree fruit) cause headache, diarrhea and Piper methysticum cause liver toxicity.[50]
Drug Interactions: Mostly patients taking drugs with a narrow therapeutic index like Cyclosporine, Digoxin, Phenytoin, Procainamide, Theophylline, Warfarin etc. should be discouraged from using herbal products. All drugs with narrow therapeutic index may either have increased adverse effects or be less effective when used in conjunction with herbal products. Ginkgo is used for Alzheimer’s disease and causes increased bleeding with aspirin. Ginseng has multiple uses and causing synergism with monoamine oxidase inhibitors. Kava is used as anxiolytic and shows synergism with benzodiazepines.
There are now many examples of the toxicity caused by the use of heavy metals in the preparations of traditional drugs. Lead, copper, mercury, arsenic, silver and gold that are commonly added to these preparations, have caused toxicity on many occasions. Patients should not use herbal drugs indiscriminately with modern medicines, as there are possibilities of drug interactions and increased risk of adverse drug reactions.
Pharmacovigilance of Herbal Medicines:
The purpose of pharmacovigilance is to detect, assess, understand and to prevent the adverse effects or any other possible drug-related problems, related to herbal, traditionally and complementary medicines.[51]Herbal medicines are widely used in both developed and developing countries however, in recent years, there are several high-profile herbal safety concerns having an impact on the public health. Herbal medicines are traditionally considered as harmless but as medicinal products they require drug surveillance in order to identify their risks. Published data shows that the risk is due either to a contaminant or to an added drug
Extremely limited knowledge about the constituents of herbal medicines and their effects in humans, the lack of stringent quality control and the heterogeneous nature of herbal medicines necessitates the continuous monitoring of the safety of these products. WHO has increased its efforts to promote herbal safety monitoring within the context of the WHO International Drug Monitoring Programme.
Various methods in pharmacovigilance are passive surveillance includes spontaneous reporting and stimulated reporting, active surveillance by sentinel sites, drug event monitoring, registries, comparative observational studies by survey study, case control study, targeted clinical investigations by investigate drug-drug interactions and food- drug interactions.[52]The importance of genetic factors in determining an individual susceptibility to adverse drug reactions is well documented and this implies to herbal medicines as well as to conventional drugs. Pharmacovigilance is therefore one of the important post-marketing safety tools in ensuring the safety of pharmaceutical and related health products .[53]
Regulatory Status of Herbal Medicines: The legal situation of herbal medicines varies from country to country. Developing countries have folk knowledge of herbs and their use in traditional medicine is wide spread. But, these countries do not have any legislative criteria to include these traditionally used herbal medicines in drug legislation.[54] Approval of herbal medicines in most countries is based on traditional herbal references, provided they are not known to be unsafe when used to treat minor illnesses. But, now-a-days claims are being made to treat more serious illnesses with herbal medicines for which no traditional knowledge is present.[55] Therefore, regulatory requirements for herbal medicines are necessary to ensure the safety, efficacy and quality and to support specific indications; scientific and clinical evidence must be acquired.[56] Depending upon the nature of herbs and market availability, different requirements exist for submission of clinical trial data and toxicity data. The regulatory requirements of herbal medicines is varies from one country to other country.[57]
IMPORTANCE OF PHARMACY PROFESSION IN PHARMACOVIGILANCE:
Role of the Pharmacist Practitioner in Pharmacovigilance-
“Safety monitoring of medicines in common use should be an integral part of clinical practice.The degree, to which clinicians are informed about the principles of pharmacovigilance, and practice according to them, has a large impact on healthcare quality. Education and training of health professionals in drug safety, exchange of information between national centres, the coordination of such exchange, and linking clinical experience of drug safety with research and health policy, all serve to enhance effective patient care. National programmes for pharmacovigilance are perfectly placed for identifying research necessary for better understanding and treatment of drug-induced diseases.”[58]
An effective approach in pharmacovigilance requires the use of modern informatics. FIP recognises that pharmacists are a key part of the post-approval environment. Also, pharmacistscan provide early detection of new ADRs and other drug related problems and identify certainpatient subgroups with exceptional sensitivities.
The changing role of the pharmacist
The position of the pharmacist within the health care system has continually beensubject to change. With respect to drug dispensing several tasks can be distinguished.[59]The pharmacist’s primary mission is to dispense drugs as prescribed by the physician and to ensure these drugs meet the required standards.[60,61]
In the literature several other ways in which the pharmacist can contribute to thesafe use of drugs are mentioned. In addition to their responsibilities relating to drug dispensing and compliance and their role in ADR reporting, which we will discuss in the next section, record keeping, education and their role regarding over the counter (OTC) products, both conventional and alternative drugs, are areas wherethey can play a prominent role.[62,63]
The pharmacist as a reporter of adverse drug reactions
This thesis specifically focuses on the significance of the pharmacist as a reporter ofadverse drug reactions. As mentioned above, in the Netherlands their contributionis substantial, which cannot besaid for the rest of the world. Not only arepharmacists not authorised to report everywhere, even where they are, theircontribution is often still relatively small.[64,65]
The contribution of the hospital pharmacist
Hospital pharmacists can also play a significant role in ADR reporting. It is in theirwork environment that the most serious adverse drug events can be seen to occur.Several recent publications have underlined the extent to which adverse drug eventsaccount for hospital admissions.This process could best be supervised by hospital pharmacists, particularly when they are directly involved in patientcare.[66] Several articles have specifically highlighted this role and have suggested that hospital pharmacists could help reduce the ADR incidence rate substantially.[67,68]
Several prerequisites need to be fulfilled to ensure that their contribution will indeed help bring down the number of adverse events and improve ADR reporting: direct involvement in patient care and a functional, widely supported hospital reporting system in whose management the hospital pharmacist should have a key role.[69,70] If hospitals were to report more, this would also enhance the surveillance of those drugs that arechiefly used in hospital settings.[69]
SURVEY QUESTIONNAIRE:
A suitable piloted self- administered survey questionnaire was designed and randomly circulated to medical practitioners of all two hospitals where the ADR reporting and monitoring system was implemented. The study questionnaire was designed to assess the attitude and perception of medical practitioners towards adverse drug reaction reporting. Few changes in the order and phrasing of the questions were made after discussion with fellow clinical pharmacists and few physicians. The final questionnaire (Table-1) consisted of ten questions and was designed specifically to answer the awareness about ADR reporting and monitoring system, its operational procedure, its usefulness, their reporting culture and also to know whether the system needs any further modification and or improvement. Questionnaire was distributed randomly to 50 medical practitioners across two study sites [TMU (n=30); SAI (n= 20)]. In order to preclude any potential bias the disclosure of name of the responder was made optional. All participants were briefed about the purpose of the study and asked to submit the filled questionnaire.
ANALYSIS:
The survey questionnaire was analyzed question wise and their percentage value was calculated. In the analysis of all questions total number of responders to questionnaire survey was considered rather total number of responders to each question. In case of unanswered questions, the number of responders unanswered to each question was categorized under ‘non responded’ category, and percentage value, question wise, was calculated.
RESULTS:
Out of 50 survey questionnaire, 40 filled questionnaires were returned giving response rate of 83.33%. The response rate from each study site was 80% from TMU and 86.66% from the Sai hospital.
According to our survey reports 80% of physicians were aware of the ADRs reporting and monitoring system in India.
Our survey results revealed that 80% [n =32 /40] of the responders were aware of existence of ADR reporting and monitoring system in India and 00% [n = 00/40] of them had reported suspected ADR to any of the pharmacovigilance centre located in India. Eighty nine percent of responders were aware of existence of ADR reporting and monitoring system at their hospital. Sixty four percent of responders had reported suspected ADR, while implemented ADR reporting and monitoring system had created awareness in 05% of the responders. The implemented ADR reporting and monitoring system has been found to be useful by 05% of responders, and 90% of the responders opined that the implemented ADR reporting and monitoring system had been benefiting the patient. Majority (05%) of responders expressed that the existing system had encouraged them to report further. Fifty percent [n = 10/20] of responders found that operating procedure of existing ADR reporting and monitoring system is simple. Zero % [n = 00/40] of responders reported to have had received proper feedback to reported reactions. Eighty five percent [n =34/40] of responders opined that pharmacist’s assistance in detection, reporting, monitoring and management of adverse drug reactions is useful. The details of attitudes and perceptions of doctors towards ADR reporting are summarised in Table-1.
Our study findings revealed several factors that influenced the doctors from reporting ADRs. Factors that encouraged ADR reporting included awareness creation, system was simple to operate, acknowledging the receipt of report, provision of feedback to the reported ADRs and constant encouragement. Factors that were considered as contributing factors for not reporting suspected ADRs included lack of time, well-known reactions, mild adverse reactions and immediate management of ADRs. Factors that were considered to be encouraging or discouraging the doctors in reporting ADR are presented in Table-2.
TABLE 1:Table Shows attitudes and perception of doctors towards the reporting.
|
QUESTIONS |
PERCENTAGES |
||
|
YES |
NO |
*NR |
|
|
80 |
15 |
05 |
and monitoring system at your hospital? |
65 |
30 |
05 |
|
00 |
90 |
10 |
|
4. Did you report any suspected adverse drug reactions to ADR reporting and monitoring system existing at your hospital? |
00 |
90 |
10 |
|
5. Has this system created an awareness of ADR reporting in you? |
05 |
85 |
10 |
|
6. Do you think that existing ADR reporting and monitoring system would benefit the patient or improve the patient care? |
90 |
10 |
00 |
|
7. Is the ADR reporting and monitoring system exists at your hospital useful for your practice? |
60 |
25 |
15 |
|
8. Is pharmacists’ assistance in detection, reporting and management of adverse drug reaction useful? |
85 |
00 |
15 |
|
9. Does the ADR reporting and monitoring system exist at your hospital encourage you to report further? |
05 |
15 |
80 |
|
10. Are you getting proper feedback to your reported reaction? |
00 |
25 |
75 |
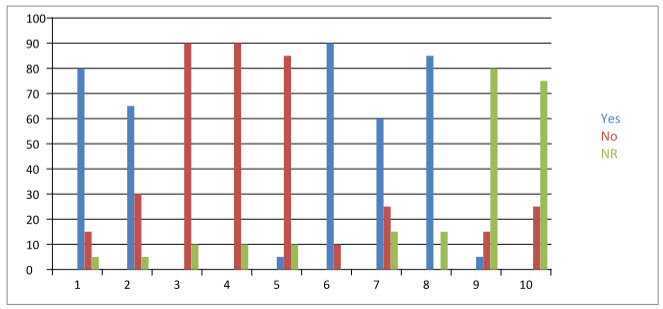
FIGURE2: CHART SHOWING PERCENTAGE OF YES AND NO IN QUESTIONNAIRE
TABLE 2: Table shows factors that encouraged of discouraged doctors from reporting an ADR.
|
FACTORS INFLUENCED
|
PERCENTAGE RESPONDERS |
|
Encouraging: (n=20) |
|
|
Creation of awareness amongst doctors |
60 |
|
Provision of feedback on reported ADR |
10 |
|
System is simple to operate |
50 |
|
Acknowledging the receipt of the report |
20 |
|
Discouraging: (n= 20) |
|
|
Time consuming |
60 |
|
Tedious |
20 |
|
Well-known reactions |
10 |
|
Mild adverse reactions |
10 |
|
Immediate management of ADRs |
20 |
DISCUSSION:
The overall results of the questionnaire survey were revealed that the doctors are aware of not only the local hospital based ADR reporting and monitoring system exists at their respective hospitals but also the national pharmacovigilance centre. Although there are several factors that either encouraged or discouraged them to report an ADR, (00%) of doctors have reported the suspected ADRs. This result suggests that ADR reporting rate may be enhanced through appropriate campaigning and overcoming the existing barriers. However, it is possible that there may be unnoticed adverse drug reactions. Unless the clinicians are trained to have a high index of suspicion, it is difficult to consider it as a part of differential diagnosis. Other reasons quoted for not reporting an ADR included no serious reactions observed, well-known reactions and reactions were managed immediately. Similar reasons for not to report an ADR was reported in one of the attitudinal survey study. This highlights the need for the encouraging medical practitioners to report suspected ADRs and therefore there is a greater potential for the pharmacists to increase the reporting rate of ADRs through creating awareness and educating the medical practitioners about the importance of reporting of ADRs.
Our study strongly suggests that there is greater need to create awareness and to promote the reporting of ADR among healthcare professionals of the country. Only such approach can greatly influence in bringing reporting culture among healthcare professionals and may improve the reporting rates of ADR in our country. Pharmacists, as doctors opined that their involvement may increase the reporting rate, have a greater role to play in the area of pharmacovigilance.
REFERENCES:
1. Rapid Pharmacovigilance Implementations In Developing Countries (Responsible Scale Up Of Treatment Programs Can Save Lives)(httpwww.rapidpharmacovigilance.org)
2. Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warning and withdrawals for prescription medications. JAMA 2002; 287: 2215-20.
3. Uppsala Monitoring Center.
4. The above quote and a lot of the above information is discussed in the new WHO report “Promoting Safety of Medicines in Children, Sep 2007.
5. Pharmacovigilance Programme of India(httpcdsco.nic.inpharmacovigilance_intro.htm
6. Pharmacovigilance Report and Analysis April 2009 (VIS00119) (httpwww.pharmaceutical-market -research. Compublications regulation _policy pharm acovigilance_report_analysis_2009.html)
7. Grootheest AC van, Edwards IR. Labelling and ‘Dear Doctor’ letters: are the noncommittal? Drug Saf 2002;25:1051-55.
8. Seligman PJ. ‘Dear Doctor…’- Evaluating the impact of risk communication efforts.Pharmacoepidemiol Drug Saf 2003; 12: 291-4.
9. Mann R, Andrews E. Pharmacovigilance. Wiley Chichester 2002. (httpdissertations.ub.rug.nlfacultiesscience2003a.c.van.grootheest)
10. National Pharmacovigilance Program (Source: National Pharmacovigilance Protocol, Ministry of Health and Family Welfare, Govt. of India (www.jipmer.edu)
11. CCox, AR., Marriott, JF., Wilson, KA.and Ferner, RE.Adverse drug reaction teaching in UK undergraduate medical and pharmacy programmes. Journal of Clinical Pharmacology, 2004; 10(4): 29:31(dspace.mak.ac.ugbitstream...okalebo-moses-cit-masters-report.pdf)
12. Yadav S. Status of adverse drug reaction monitoring and pharmacovigilance in selected countries. Indian J Pharmacol 2008; 40: 4-9.(www.ijp-online.com)
13. Pharmacovigilance in the pharmaceutical industry; J C C Talbot and B S Nilsson; Br J Clin Pharmacol; 1998 May; 45(5): 427–431.( httpwww.chillibreeze.com articles_ various Pharmacovigilance.asp)
14. Food and Drug Administration. (1996). The clinical impact of adverse event reporting. A MedWatch continuing education article, Retrieved November 26, 2005; from http://www.fda.gov/medwatch/:
15. DOH. (1998). National Reporting System of Adverse Events in Taiwan. A journal of public health, Jinhua St. , Taipei , 100, Taiwan: (pp 19-21)
16. World Health Organization.(2002). The importance of pharmacovigilance. International conference on pharmacovigilance, Geneva.
17. How To Report Adverse Drug Reactions.Sited on(www.livestrong.com.mht)
18. ADR Form. (www.jipmer.educharu.pdf)
19. Central Drugs Standard Control Organization. Available from: http://www.cdsco.gov.in.
20. Dahanukar SA, Thatte UM. Ayurveda revisited. 1 st ed. Mumbai: Popular Prakashan Private Ltd.; 1989.
21. Dahanukar SA, Thatte UM. Ayurveda unravelled. New Delhi: National Book Trust; 1996.
22. Ganesh Krishna Gadre, "13 th Adhyaya" Ashtang Hridaya. 2 nd ed. Pune: Aryabhushan Printing Press; 1910; p. 65.
23. Available from: http://nccam.nih.gov/health/ayurveda/ (Last Accessed on 2007 September 7).
24. Thatte UM, Rege NN, Phatak S, Dahanukar SA. The flip side of ayurveda? J Postgrad Med 1993; 39: 179-82.
25. Gogtay NJ, Bhatt HA, Dalvi SS, et al . The use and safety of non-allopathic Indian medicines. Drug Saf 2002; 25: 1005-19.
26. Acharya Jadavji Trikramji, "8 th Adhyaya" Charak Samhita. 5 th ed. Varanasi: Chaukhambha Sanskrit Sansthan; 2001; p. 275.
27. Acharya Jadavji Trikramji, editor. "8 th Adhyaya" Charak Samhita. 5 th ed. Varanasi: Chaukhambha Sanskrit Sansthan; 2001; p. 276.
28. Pndit Kashinath Shastri, "2 nd Adhyaya" Rasantarangini. 11 th ed. Neaw Delhi: Sri Jainendra Press; 1994; p. 22-4.
29. Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al . Heavy metal content of ayurvedic herbal medicine products. JAMA 2004; 292: 2868-73.
30. Parab S, Kulkarni RA, Thatte UM. Heavy metals in herbal medicines. Indian J Gastroenterol 2003; 22: 111-2.
31. Acharya Jadavji Trikramji, "1 st Adhyaya" Charak Samhita. 5 th ed. Varanasi: Chaukhambha Sanskrit Sansthan; 2001; p. 23.
32. Available from: http://indianmedicine.nic.in/html/edu/aemain.htm (Last Accessed on 2007 September 7).
33. Available from: http://indianmedicine.nic.in/html/ayurveda/asmain.htm (Last Accessed on 2007 September 7).
34. Malik V, Drugs and Cosmetic Act, 1940, "4 th Chapter", Part I, 14 th ed. Lucknow: Eastern Book Company; 2002; p. 37-44.
35. Dahanukar SA, Thatte UM. Can we prescribe ayurvedic drugs rationally? Indian Pract 1998; 51: 882-6.
36. Available from: http://indianmedicine.nic.in/html/manufactures/manufactuer.htm#ad (Last Accessed on 2007 September 7).
37. Parab SM, Kalele SS, Verma S, Kulkarni RA, Shinde VJ, Bhalerao SS, et al . Safety issues with medicines from alternative system of medicine. In: Proceedings of Workshop on Pharmacovigilance: Promoting Drug Safety through Collaboration; 2003 December 5-6, Parel, Mumbai: Seth GS Medical College. p. 22.
38. Available from: http://indianmedicine.nic.in/html/pharma/apmain.htm (Last Accessed on 2007 September 7).
39. Available from: http://203.200.90.6/tkdl/LangDefault/common/Abouttkdl.asp?GL = Eng (Last Accessed on 2007 September 7).
40. WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems, World Health Organization, Geneva, 2004
41. S Z Rahman & K C Singhal, Problems in pharmacovigilance of medicinal products of herbal origin and means to minimize them, Uppsala Reports, WHO Collaborating Center for ADR monitoring, Uppsala Monitoring Centre, Sweden, Issue 17 January 2002; 1-4 (Supplement)
42. Barnes J, Mills SY, Abbot NC, Willoughby M, Ernst E. Different standards for reporting ADRs to herbal remedies and conventional OTC medicines: face-to-face interviews with 515 users of herbal remedies. British Journal of Clinical Pharmacology 1998; 45(5): 496-500.
43. Ang-Lee MK, Moss J, Yuan C. Herbal medicines and perioperative care. Journal of the American Medical Association 2001; 286: 208-216.
44. Guidelines for the assessment of herbal medicines, WHO/TRM/91.4, WHO Geneva,1991
45. Guidelines for safety monitoring of herbal preparations are under development.(apps.who.int)
46. Winslow LC, Kroll DJ, Herbs as medicines, Archives of Internal Medicine,158, 1998; 2192-2199 (www.ijpsr.com)
47. Gossell M, Simon OR, West ME, The past and the present use of plants for medicines, West Indian Medical Journal, 55, 2006; 217.( www.ijpsr.com )
48. De-Smet PGAM, The role of plant derived drugs and herbal medicines in health care, Drugs, 54, 1997; 801-840.
49. WHO technical report series, Guidelines for the assessment of herbal medicines, 863, 1996; 178-184.
50. Kuhn MA, Herbal remedies: drug-herb interactions, Critical Care Nurse, 22, 2002; 22-32.
51. Routledge P, 150 years of pharmacovigilance, The Lancet, 351, 1998; 1200-1201
52. Bigoniya P, Pharmacovigilance of herbal medicines: current status and future strategies, The Pharma Review, 5, 2009; 77-88.
53. Chan TYK, Monitoring the safety of herbal medicines, Drug Safety, 17, 1997; 209-215.
54. Sukhdev S, Arun N, Kalia AN, Patentability of herbal products: a review, The Pharma Review, 4, 2008; 118-124.
55. Raskin L, Ribnicky DN, Komarnytsky S, Lic N, Poulev A, Borisjuk N, Plants and human health in the twenty first century, Trends in Biotechnology, 20(12), 2002; 522-531.
56. Mukherjee PK, Exploring botanicals in Indian systems of medicine regulatory perspectives, Clinical Research and Regulatory Affairs, 20, 2003; 249-264.
57. Calixto JB, Efficacy, safety, quality control, marketing, and regulatory guidelines for herbal medicines, Brazilian Journal of Medical and Biological Research, 33, 2000, 179-189.
58. WHO, “The Importance of Pharmacovigilance, 2002”(www.fip.org)
59. Brouwers JRBJ, Roon EN van, Toering DJ. De ziekenhuisapotheker als farmacotherapeut [The clinical pharmacist as pharmacotherapist]. Pharm Weekbl 2003; 138: 692-6
60. Clause S, Fudin J, Mergner A, Lutz JL, Davanaugh MM, Fessler K, Chirumamilla S.Prescibing privileges among pharmacists in Veterans Affairs medical centres. Am J Healt-SystPharm; 58: 1143-5.
61. Grootheest AC van, Puijenbroek EP van, Jong – van den Berg LTW de. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf(in press).
62. Poston J, Parish P. The pharmacists. In: Monitoring for drug safety (WHW Inman, editor). MTP Press.Lancaster 1986.
63. Major E. The yellow card scheme and the role of pharmacists as reporters. Pharm J 2002; 269: 25-6.
64. . Olsson S. National Pharmacovigilance Systems – Country Profiles and Overview (second edition). Uppsala Monitoring Centre. Uppsala 1999.
65. Kelly B. Pharmacovigilance: more a responsibility than a role. Austr Pharm 2001;20:128
66. Anonymus. Top-priority actions for preventing adverse drug events in hospitals. Recommendations of a expert panel. Am J Health-Syst Pharm 1996; 53: 747-751.
67. Leape LL, Cullen DJ, Dempsey Clapp M, Burdick E, Demonaco HJ, Erickson JI, Bates DW. Pharmacist Participation on Physician Rounds and Adverse Drug Events in the Intensive Care Unit. JAMA 1999; 282: 267-70.
68. Bemt PMLA van de. Drug Safety in Hospitalised Patients. Thesis Rijksuniversiteit Groningen.Groningen 2002.
69. Major E. The yellow card scheme and the role of pharmacists as reporters. Pharm J 2002; 269: 25-6.
70. Ahmad SR, Freiman JP, Graham DJ, Nelson RC. Quality of Adverse Drug Experience Reports Submitted by Pharmacists and Physicians to the FDA. Pharmacoepidem Drug Saf 1996; 5: 1-7.









