About Authors:
Ali K. Akhtar, Waquar A. Khan,Lubna azmi
Faculty of Pharmacy,
Integral University Lucknow,
India
Abstact:
The broad and potent activity of benzofuran has established it as one of the biological importent scaffold. This article is covered the methods of synthesis of benzofuran and its derivatives, structural activity relationship and pharmacological activities so it is an effort to highlight the importance of the benzofuran in the present context and promise they hold for the future.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1185
INTRODUCTION
The Benzofuran containing structures have been found among naturally occurring furocoumarins, such as psoralen and methoxalen isolated from the seed of Ammi majus L. and used for the treatment of psoriasis and other dermal diseases[1, 2] The (Benzofuran-2-yl) carbinols exhibit various biological activities. Such derivatives were investigated as antibacterial3] or antifungal agents[3, 4]. Moreover, optically active 2-(2-tert-butylamino-1-hydroxyethyl)benzofurans were investigated as β-blockers[5] 2-Substituted benzofurans can inhibit the HIV-1 reverse transcriptase[6]or act as antiaging compounds[7]. A previous literature survey described a variety of 2- substituted benzofuran derivatives that received a great deal of attention for their anti-HIV-1[8,9,10,11], anticancer[11,12,13,14,15], and antimicrobial[11,16,17,18], activities.The derivatives of keto benzofuran are useful as medicines, such as amiodarone and benziodarone, particulary for the treatment of athological syndromes of the cardiovascular system, such as arrhythmia[19]. Several aminobenzofuran derivatives likewise exhibited a marked antiarrhythmic activity[20]. Moreover, benzofuranyl methanols have been reported as hypolipidemic agents[21]. For example, 1-[(benzofuran-2-yl)phenylmethyl]imidazoles had a potent, reversible, non-selective aromatase-inhibitory effect[15], 2-{4-[(benzofuran-2- yl)carbonyl]piprazin-1-yl}-3-propylpyridine exhibited good anti-HIV activity[10] and 2-(benzimidazol- 2-yl-carbonyl) benzofuran derivative had a strong inhibitory activity against Candida albicans Nmyristoyl-transferase[18].
CHEMISTRY OF BENZOFURAN:
The benzene ring is fused with five member heterocyclic ring i.e. furan and formed bicyclic ring benzofuran. It also called as Coumarone and obtained from salicylaldehyde by conversion into the aryloxyacetic acid by reaction with sodium chloroacetate in the presence of alkali followed by cyclisation with a mixture of acetic anhydride, acetic acid and sodium acetate. The reactions proceed by an internal Perkin’s reaction followed by decarboxylative dehydration[22].
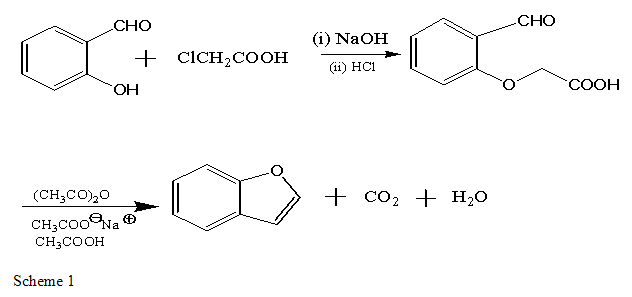
EXPERIMENTAL PROCEDURE
o-Formylphenoxyacetic acid.A solution of 80.0 g. (2 moles) of sodium hydroxide pellets in 200 ml. of distilled water is added to a mixture of 106 ml. (122 g., 1 mole) of salicylaldehyde (Note 1), 94.5 g. (1 mole) of chloroacetic acid and 800 ml. of water. The mixture is stirred slowly and heated to boiling. The resulting black solution is heated under reflux for 3 hours. The solution is acidified with 190 ml. of concentrated hydrochloric acid (sp. Gr. 1.19) and is steam-distilled to remove unchanged salicylaldehyde (40.0–40.5 g.). The residual acidic mixture is cooled to 20°, and the precipitated product is collected on a Büchner funnel and rinsed with water. The light tan solid when dry weighs 99–100 g. (82–83% based on recovered salicylaldehyde), m.p. 130.5–133.0°C [22-24].
B. Coumarone. A mixture of 90.0 g. (0.5 mole) of crude, dry o-formylphenoxyacetic acid, 180 g. of anhydrous, powdered sodium acetate, 450 ml. of acetic anhydride, and 450 ml. of glacial acetic acid in a 2-l. flask is heated under gentle reflux with stirring for 8 hours. The hot black solution (total volume ca. 1.2 l.) is poured into 2.5 l. of ice water and extracted with one 600- ml. portion of ether. The ether layer is washed with one 600-ml. portion of water and then with several portions of cold dilute 5% sodium hydroxide solution until the aqueous layer is basic. The ether layer is washed successively with water and saturated sodium chloride solution and is partially dried over anhydrous granular sodium sulfate. The ether is removed at water-bath temperature and the product is distilled, b.p. 166.5–168.0° (735 mm.) or 97.5–99.0° (80 mm.). The water-white benzofuran weighs 37.5–40.0 g. (63.5–67.8%, 52–56% overall from salicylaldehyde), n20D 1.5672; λmax 245 (log ε 4.08), 275 (3.45), and 282 mμ (3.48)[22-24].
[adsense:468x15:2204050025]
SYNTHESIS OF BENZOFURAN DERIVATIVES
A. 3-ETHOXYCARBONYL BENZOFURANS
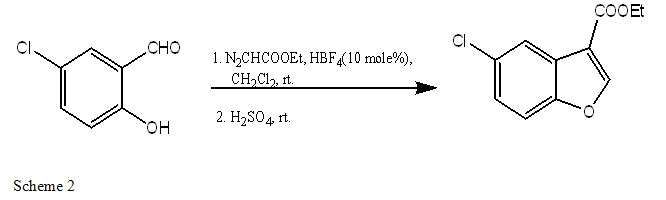
We can use various substituted aromatic aldehyde viz. 5- chlorosalicylaldehyde, 3, 5- Dichloro-salicylaldehyde, 3- methoxysalicylaldehyde, etc.and form mono or di-substituted 3-ethoxy-carbonyl benzofuran derivatives[25].
B. 3- METHYL BENZOFURAN DERIVATIVES
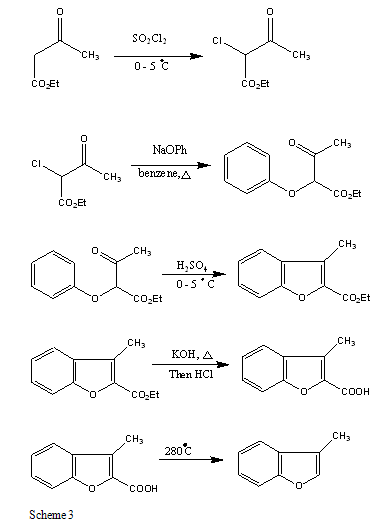
The Methyl benzofuran synthesized by four step reaction from ethyl α-chloroacetoacetate and sodium phenolate and its yield is 84 – 88 % [26].
C. BENZOFURAN-3 ACETIC ACID DERIVATIVES
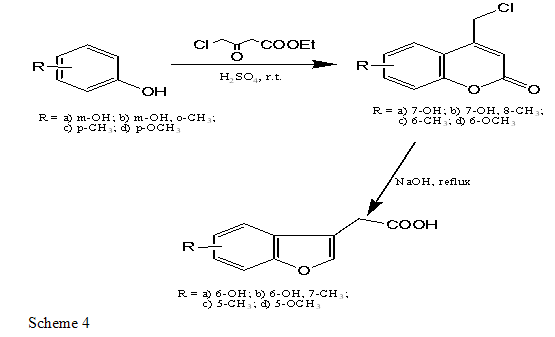
The phenol is treated with ethyl 4-chloroacetate in presence of H2SO4 acid and formed 4-chloromethylcoumarin, and which is reflux with NaOH and benzofuran 3-acetic acid[27].
D. 3-METHYL, 5-HYDROXYBENZOFURAN DERIVATIVES
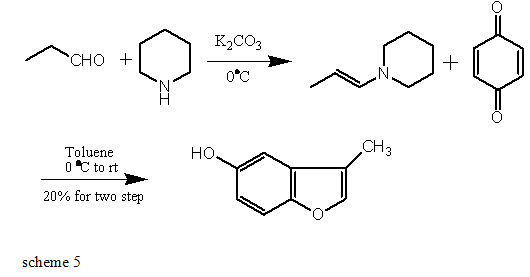
The Benzofuran scaffold was constructed by Michael addition of quinone with activated propanal and then cyclization. It seemed that piperidine is the best amine to the preparation of such compound[28-29].
E. 3-ETHYLCARBOXYLATE BENZOFURAN DERIVATIVES
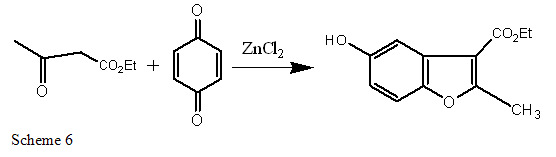
The Benzofuran scaffold was constructed by Michael addition of quinone with ethyl acetyl acetate and then cyclization.Such types of derivatives are prepared by the catalysis of anhydrous zinc chloride[29-30].
F. A STRAIGHTFORWARD SYNTHESIS OF BENZOFURAN DERIVATIVES
The reported synthesis starting from 1-trimethylsilyl-1,3- butadiyne A [31] and protected 2-iodophenol B, we have realized the synthesis of several ortho-substituted aryl diynes C,which produce various benzofuran derivatives.
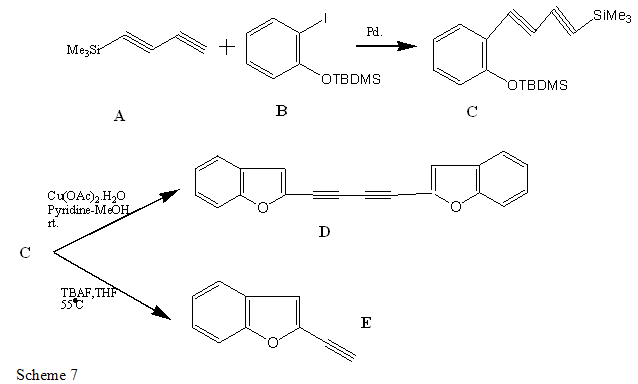
The reaction of compound C with Cu (OAc)2.H2O and directly obtained the bis-benzofuran derivatives D with the two ring linked to the position 2,2’by two triple bonds[35].The Compound C with a fluoride source, TBAF[32-33] in at 55 ?C, led to the heterocyclic product, 2-ethynyl-benzofuran E[34-35].
G.
1.SYNTHESIS OF 2-(4-METHOXYPHENYL)-5-METHYLBENZOFURAN
To a solution of 2-((4-methoxyphenyl)ethynyl)-4-methylphenyl acetate in methanol was added potassium carbonate . The reaction mixture was heated to 60 _C overnight, then being left to cool. The reactionwas poured onto EtOAc,washed with brine, and dried over anhydrous sodium sulfate. Purification was performed by flash chromatography, and an amorphous solid was obtained. Yield 90%;
2.GENERAL PROCEDURE FOR (3,5-DIMETHOXYPHENYL)(2-(4-METHOXYPHENYL)-
5-methylbenzofuran-3-yl)methanone
SnCl4 (1.2 eq.) was added dropwise to a mixture of 5 (50 mg,0.21 mmol) and the 3,5-dimethoxybenzoyl chloride (1.2 eq.) in drydichloromethane and the resulting solution was stirred at roomtemperature overnight. The reaction was quenched with ice andstirred for 1 h. The organic layer was separated and the aqueous layer was extracted with dichloromethane. The combined organic extracts were washed with brine, dried over sodium sulfate. Purificationwas performed by flash chromatography, and the yellowoil was obtained[67]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SPECTROSCOPY OF BENZOFURAN
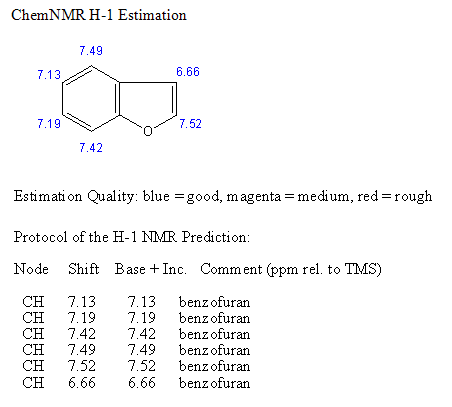
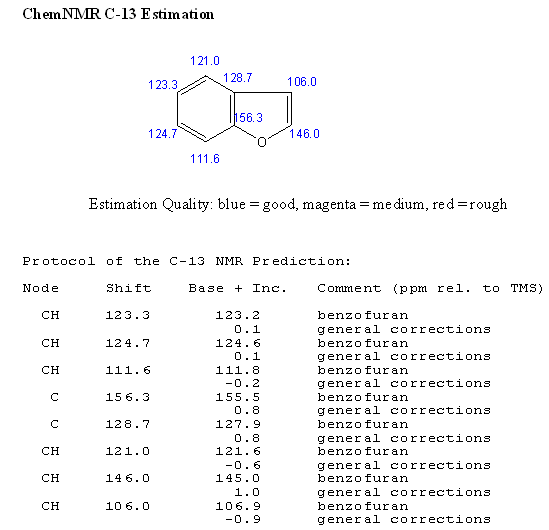
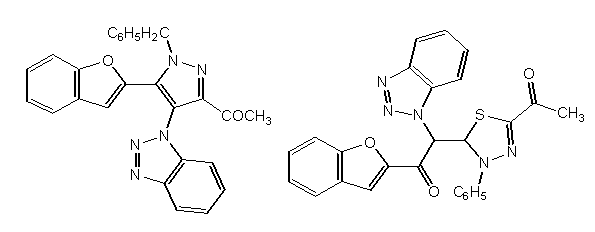
STRUCTURAL ACTIVITY RELATIONSHIP OF BENZOFURAN DERIVATIVES
1. The thiadiazole derivative of benzofuran will the most potent anti-inflammatory compound after 2 h and it showed also reasonable anticonvulsant activity in ScMet without neurotoxicity. The pyrazole ring system is more active than the 1, 3, 4-thiadiazole one[36].
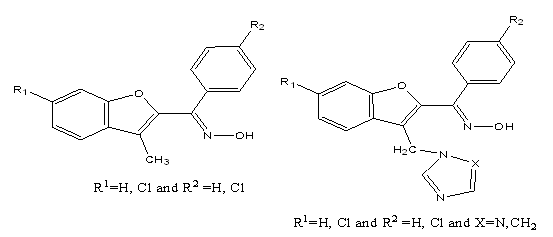
2. The aryl benzofuryl ketoxime moiety with azole residue—both with proven antimycotic activity and gave satisfactory increases in activity introduced alone—in a single molecule did not give the desired successful activity values[37].
3. The pyrazole derivative of benzofuran showed a higher antinociceptive effect than all other heterocycles [36].
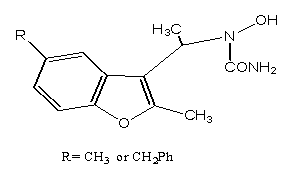
4. The N-alkyl-N-hydroxyureas are also capable of inhibiting the synthesis of leukotrienes. The Benzofuran derivatives containing hydroxyurea fragments at position 3 of the benzofuran ring will show anti-inflammatory activity[38].
5. The 1-(thiazol-2-yl) pyrazoline derivatives of benzouran showed excellent activity against Gram-negative bacteria (inhibitory zone 25 mm), good activity against Gram-positive bacteria (inhibitory zone 20 mm)[39].
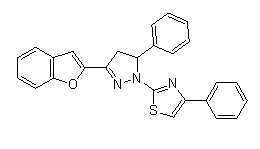
6. All following compounds showed inhibition zones and therefore antifungal activities against C. albicans more than the reference sample Flucanazole, while compound showed similar antifungal activity against C. albicans[39].
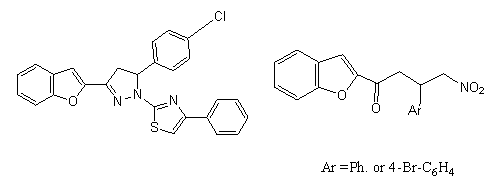
7. It can be concluded that benzofuran, pyrazoline and thiazole moieties are essential for the antimicrobial activity[39].
8. The anti-inflammatory activity of arylalkanoic acids derivatives of benzofuran is due primarily to their ability to inhibit cyclooxygenase and thus to disrupt prostaglandin biosynthesis[27].
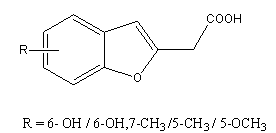
9. The phenyl ketone without a substituent on the benzene ring showed relatively weak cytotoxicity, its derivatives, which has a methoxy group at the ortho position were moderately active as a anti-tumor agent[40].
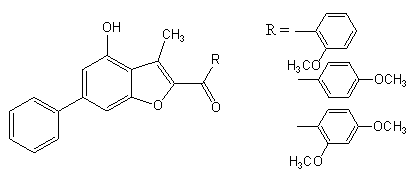
10. The methoxy group at the para position also improved the biological activity to some extent. The two methoxy groups at the ortho and para positions was the most potent anti- tumour benzofuran derivative[40].
11. The 4-amino-3-butyl-2-thioxo-2,3-dihydro-thiazole-5-carboxylic acid-(1-benzofuran-2-yl-ethylidene)- hydrazide will to have moderate in vitro anti-HIV-1 activity[11].
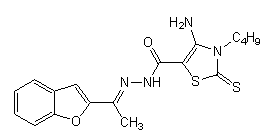
The 2-[(1- benzofuran-2-yl-ethylidene) hydrazono]-3-phenyl-thiazolidin- 4-one has a larger antiviral cytopathic effect[11].
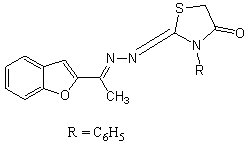
The substituted thiazolidinon-2-ones increased the anti-HIV potency. The above compound (R = phenyl) had the highest activity, which was decreased when R = n-butyl groups was separated by one carbon atom spacers[11].
12. The p-tolyl substituted thiazole derivatives of benzofuran decreased the growth of breast and lung cancer to some extent compared with compound (R = benzyl), which was completely inactive.
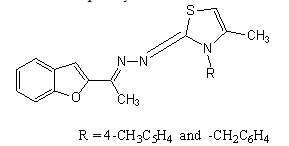
The thiazolo [4,5-d] pyrimidines, showed weak activity against breast and lung cancer[11].
13. The Amiodarone, an iodinated lipophilic benzofuran derivative is widely used in the treatment of ventricular tachyarrhythmia and atrial fibrillation[41-42].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHARMACOLOGICAL ACTIVITY
Anti- Cancer activity:
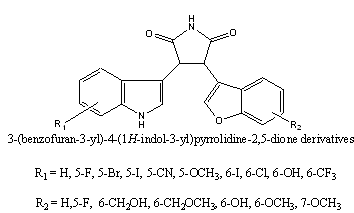
a. A library synthesis of 4-hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives and exhibited selective cytotoxicity against a tumorigenic cell line[43]. Some 2-arylbenzofurans[44] are inhibitors of cell proliferation and plateletactivating factor, and some of them show good fungicidal, insecticidal, and cytotoxic activities. Therefore, 2-arylbenzofurans have been a new subject of synthetic studies for the development of biologically active compounds[44]. Lobaric acid has been isolated from lichen, Stereocaulon sasakii together with a new benzofuran, sakisacaulon A. Lobaric acid inhibited the polymerization of tubulin[45]. Structure–activity relationship of lobaric acid and its derivatives on inhibitory activity of tubulin polymerization was discussed[45]. The benzofuran-3-yl-(indol-3-yl)maleimides[46] potent GSK-3β inhibitors. Some of these compounds show picomolar inhibitory activity toward GSK-3β and an enhanced selectivity against cyclin-dependent kinase 2 (CDK-2). Selected GSK-3β inhibitors were tested in the pancreatic cancer cell lines MiaPaCa-2, BXPC-3, and HupT3. The present data suggest a possible role for GSK-3β inhibitors in cancer therapy, in addition to their more prominent applications in CNS disorders[46].
b. The new 2-arylbenzofuran derivatives, cathafurans A, B, C and D [fig.7] were reported which are isolated from the stem bark of Morus cathayana[47]. Their structures were determined by spectroscopic methods. Cathafurans Band Cexhibited moderate activities against five human cancer cell lines, with IC50 values ranging from 6.17 to 9.60 μg/mL[47]. Some new Benzofuran derivatives[48] were reported against twelve different human cancer cell lines and all of the compounds were more potent than the comparative standards [48]. The (3-Amino-6-thiophen-2 -yl-thieno [2,3-b] pyridin-2-yl) phenyl-methanone was discovered as a new type of cytotoxic agent selective against a tumorigenic cell line[40]. The benzo[b]thiophene and benzofuran combretastatin analogues and their phosphate prodrugs for their high antitumor activity in In vitro and in vivo models. Cell exposure to IC50 of derivatives and CA-4 led to the arrest of various cell types In the g2/m phase of the cell cycle and induction of apoptosis. Mainly, benzofuran combretastatin analogues induced the formation of Multinucleated cells with abnormal chromatin distribution, with only a minimal effect on the microtubule Organization[49]. CC-1065 analogues bearing different DNA-binding subunits were synthesized. A terminal C5- NO2 and -F moiety at the DNA-binding subunit increased the drug’s potency and antitumor efficacy[50].
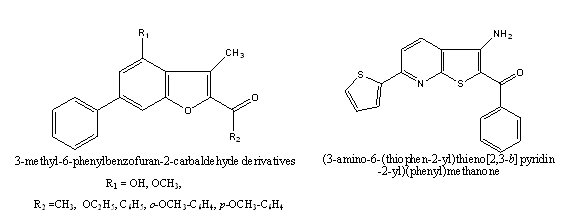
Anti-histaminic activity
The H3 receptor antagonists based on a 2-aminoethylbenzofuran skeleton [fig.10] have been discovered, which are potent in vitro at human and rat H3 receptors. One compound in particular, 4-(2-[2-(2(R)-methylpyrrolidin-1- yl) ethyl] benzofuran-5-yl) benzonitrile (ABT-239), combined potent and selective H3 receptor antagonism and excellent pharmacokinetic and metabolic properties across species, with full efficacy in two behavioral models. The potency and selectivity of this compound and of analogues from this class support the potential of H3 receptor antagonists for the treatment of cognitive dysfunction[51].A facile and scaleable synthesis of a potent (benzofuranoid)and selective histamine H3 receptor antagonist, ABT-239 was developed starting from commercially available 4’-sshydroxy-biphenyl-4- carbonitrile[52].
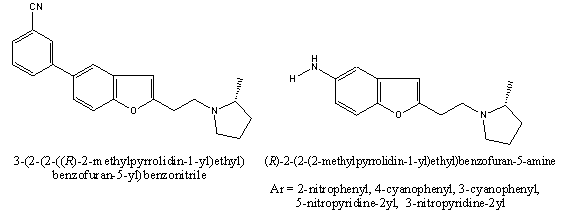
One compound in particular, the {2-[2-(2-(R)-methylpyrrolidin-1-yl)ethyl]benzofuran-5-yl}(5-nitropyridin-2-yl)amine, gave the best binding potency (human Ki of 0.05 nM, rat Ki of 0.11 nM), which represented a 9-fold (in human) and an 11-fold (in rat) improvement over ABT-239, a compound previously reported to have excellent in vitro potency and in vivo efficacy[53].
Anti-Depression
Selective serotonin reuptake inhibitors (SSRIs) have achieved great success in treating depression and related conditions. SSRIs act by blocking the neuronal reuptake of serotonin (5-HT), increasing the concentration of synaptic 5-HT. Several benzofuran derivatives linked to a 3-indoletetrahydropyridine[54] through an alkyl chain were prepared and evaluated for serotonin transporter and 5-HT1A receptor affinities[54].
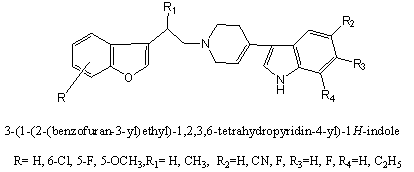
Antipsychotic Agents
The latter has high affinity for D2 and 5-HT1A receptors, whereas it possesses only a weak affinity for 5-HT2A sites. In cellular models of signal transduction, this following compound behaves as a silent antagonist at rD2 receptors while activating h5-HT1A receptors with an efficacy at least equivalent to that of the prototypical 5-HT1A agonist (±)-8-OH-DPAT. These dual actions confer a unique pharmacological profile to the product. In a behavioral model predictive of positive symptoms, this compound have an activity comparable to that of the typical antipsychotic haloperidol, while it is devoid of cataleptogenic effects. Although it produces behaviors characteristic of 5-HT1A receptor activation in rats, these occur at doses 100 times higher than those with (±)-8-OH-DPAT. We believe that the relative balance of D2 and 5-HT1A actions in this compound is appropriate, possibly optimal, to ensure superior efficacy and tolerability over existing antipychotic drugs[55].
For Alzheimer’s disease
The new hybrid molecules linking a benzofuran ring to a N-methyl-N-benzylamine through a heptyloxy chain, affording a series of potential multifunctional drugs for AD. The cholinesterase inhibitory activity was extended to the inhibition of Aβ fibril formation for benzofuran derivatives[56]. The benzoyl moietyshowed an additional neuroprotective effect. The Benzofuran-Based Hybrid Compounds have the Inhibition of Cholinesterase Activity, βAmyloid Aggregation, and AβNeurotoxic activities[56]. A novel series of benzofuran derivatives as potential positron emission tomography (PET) tracers targetingamyloid plaques in Alzheimer’s disease (AD) were synthesized and evaluated. In vitro fluorescent labeling of AD sections with compound intensely stained not only amyloid plaques, but also neurofibrillary tangles. The 11C of labeled compound [11C] was prepared by reacting the normethyl precursor, 5-hydroxy-2-(4- aminophenyl)benzofuran, with [11C] compound methyl triflate. In addition, this PET tracer showed in vivo amyloid plaque labeling in APP transgenic mice. Taken together, the data suggest that a relatively simple benzofuran derivative, [11C], may be a useful candidate PET tracer for detecting amyloid plaques in the brains of patients with Alzheimer’s disease[57].
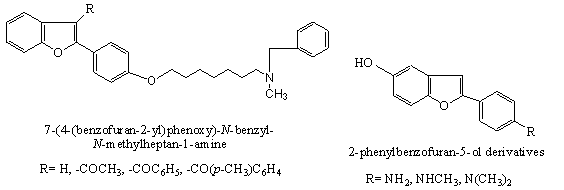
Anti-Protozoal
a. The effects of the substitution on the amidine groups, varying the placement of cationic moieties,altering the overall geometry of molecules by changing the position of the benzofuran fragment, and the elongation of the aliphatic linker between aromatic rings from three to six carbon atoms on antiprotozoal properties of novel pentamidine analogues[58]. The results of the in vitro evaluation of dications of compounds against bloodstream form trypomastigotes of T. b. rhodesiense (STIB900), chloroquine resistant P. falciparum (K1), axenic amastigotes of L. donovani (MHOM/SD/62/ 1S-CL2D), and their cytotoxicities against rat myoblast cells(L6) are evaluated and compared to the activity of pentamidine[58].
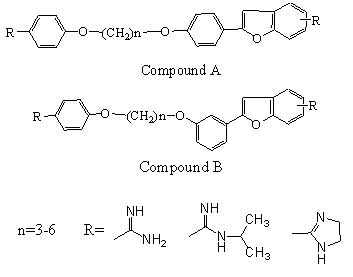
b. A series of cationically substituted 2-phenylbenzofurans[59]have been synthesized, and their in vitro antiprotozoal properties against Trypanosoma brucei rhodesiense, Plasmodium falciparum, and Leishmania donoVani, as well as cytotoxicity against mammalian cells, have been evaluated. Introduction of methoxy or hydroxy groups in the 7- and/or 2′-position afforded derivatives that were highly selective against T. b. rhodesiense, P. falciparum, and L. donoVani[60]. The bisbenzofurans displayed activity against L. donoVani superior to that of pentamidine[60] was reported.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Anti-Microbial
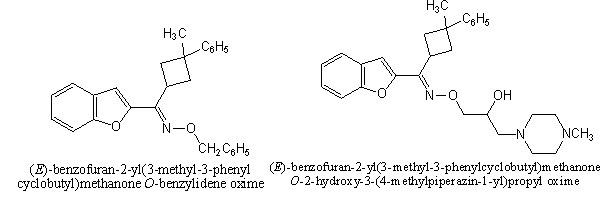
a. The HIV-1 RT inhibitory activity showed that compounds[48] were reported potent but with lower therapeutic index than Atevirdine. The HCV NS3-4A protease inhibitor activity of the tested compounds revealed that they have weaker potency and less therapeutic index than VX-950, although compounds A, B and C respectively exhibited significant activity[48]. The synthesized compounds (E )-1-(1-benzofuran-2-yl)-2-mesitylethanone- O-benzoyl oxime[61]was reported the most active derivative against S. aureus ATCC 6538 and E. coli ATCC 25922. The other compounds exhibited moderate activity against the other test microorganisms[61]. The compounds (benzofuran-2-yl)(3-phenyl-3-methylcyclobutyl)-O-[2-hydroxy-3-(Nmethylpiperazino)] Propylketoxime21was also the most active derivative against S. Aureusatcc 6538. Similarly (benzofuran-2-yl)(3-phenyl-3- Methylcyclobutyl)-O-benz- ylketoximeshowed good antimicrobial effect against C. Albicans[16].
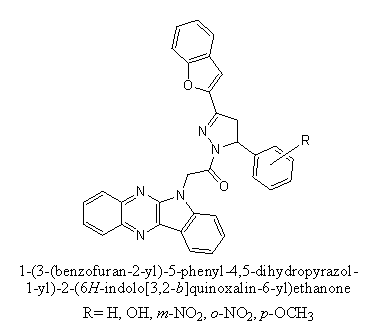
b. The benzofuran analogues[62] were reported good antibacterial activity and MICs were found bellow 10 lg/mL against Escherichia coli, Pseudomonas aeruginosa and Streptococcus aureus, which can compared with sparfloxacin and norfloxacin[62].
c. The 2-Methyl-3- nitrobenzofuran and analogs containing 7-N02, 5-N02, 7-Br, 7-CONH2, and 7-CF3 substituents are bacteriostatic. The spectrum of activity of these compounds is similar to nitrofurazone; however, a strain of E. coliBr which has increased resistance to nitrofurazone did not show increased resistance to 3, 7-dinitro-2-methylbenzofuran[63]. The C-4 side chain modification of 3-methylbenzofuran-2-carboxylate derivatives[64] was reported in the identification of a potent and selective Candida albicans N-myristoyltransferase (CaNmt) inhibitor RO-09-4609, which exhibits antifungal activity against C. albicans in vitro. Further modification of its C-2 substituent has led to the discovery of RO-09-4879, which exhibits antifungal activity in vivo[64].
Cardiovascular effects
Recent developments in antiarrhythmic therapy have indicated that the best approach to pharmacologically controlling supraventricular arrhythmias and life-threatening ventricular tachyarrhythmias is by prolonging cardiac repolarization rather than by blocking conduction. a new series of carboxymethoxybenzoyl and benzyl derivatives of benzofuran has been prepared and evaluated as thyroid hormone receptor antagonists. Within this series, 2-methyl-3-(3, 5-diiodo-4-carboxymethoxybenzyl)benzofuran KB130015 was found to reveal the most promising in vitro data. It inhibits the binding of 125I-T3 to the human thyroid hormone receptors (hThR) α1 and β1. T3-Antagonism was confirmed in reporter cell assays employing CHOK1 cells (Chinese hamster ovary cells) stably transfected with hThR α1 or hThRβ1 and an alkaline phosphatase reporter gene downstream a thyroid response element[65]. Various 5-aminobenzofuran derivatives were prepared from khellin and screened intravenously in the dog for their potential antiarrhythmic activity against ouabain-induced ventricular arrhythmia and in the Harris test. The two long-acting derivatives N-[4, 7- dimethoxy-6-(2-pyrrolidinoethoxy)-5-benzofuranyl]-N'-methylure and N-[ 4,7-dimethoxy-6-( 2-piperidinoethoxy)- 5-benzofuranyl]-N'-methylurea showed advantages when compared to quinidine and disopyramide[66]
Hypoglycemic activity
Mulberrofuran U (2) and moracin M-3-O-beta-D-glucopyranoside (3) were isolated, along with six known compounds [beta-sitosterol, beta-sitosterol-3-O-beta-glucopyranoside, ursolic acid, moracin M (1), kaempferol-3-O-beta-glucopyranoside and quercetin-3-O-beta-glucopyranoside] [68],They have high activity.
Conclusion:
The article has outlined the chemistry and pharmacological activity of the benzofuran scaffold. The synthetic methodologies indicate the simplicity,and vesatility which of medicinal chemist a complete range of novel derivatives.
The high degree of protection against fungal infection can be positives sign for further investigation of benzofuran derivatives as anticancer and their antiprotozoal activities the broad spectrum antibacterial and anti-fungal activity of compounds could lead to a new series of antimicrobials. The benzofuran derivatives have demonstrated significant antiarrhythmic activity, antidepression and antipsychotic activity. The histamine receptor against action of these derivatives further their biological importance.
Thus benzofuran scaffold is not only synthetically important but also posseses a wide range of promising pharmacological activity. Future investigations of this scaffold could give some more encouraging result.
REFERENCES:
1. J.C. Gonzalez-Gomez, L. Santana, E. Uriarte, A furan ring expansion approach to the synthesis of novel pyridazino-psoralen derivatives, Tetrahedron 61 (2005) 4805-4810.
2. P. Nore, E. Honkanen, A new synthesis of methoxalen, J. Heterocycl. Chem. 17 (1980) 985-987.
3. Bevinakatti, H. S.; Badiger, V. V. Arch. Pharm. (Weinheim) 1981, 314, 162.
4. Wahbi, Y.; Caujolle, R.; Tournaire, C.; Payard, M.; Linas, M. D.; Seguela, J. P. Eur. J. Med. Chem. Chim. Ther. 1995, 30, 955.
5. Machin, P. J.; Hurst, D. N.; Osbond, J. M. J. Med. Chem. 1985, 28, 1648.
6. Hoffman, J. M.; Smith, A. M.; Rooney, C. S.; Fisher, T. E.; Wai, J. S.; Thomas, C. M.; Bamberger, D. L.; Barnes, J. L.; Williams, T. M. J. Med. Chem. 1993, 36, 953.
7. Knoll, J. CNS Drug Rev. 2001, 7, 317.
8. Varvaresou, A., Iakovou, K., Filippatos, E., Souli, C., Calogeropoulou, T., Ioannidou, I., Kourounakis, A. P., Pannecouque, C., Witvrouw, M., Padalko, E.; Neyts, J., De Clercq, E., and Tsotinis, A., Synthesis, Anti-retroviral and antioxidant evaluation of a series of new benzo[b]furan derivatives. Arzneim. Forch., 51, 156-162 (2001).
9. Vacca, J., Lin, J., Yeh, K., Chodakewitz, J., Deutsch, P., and Ju, W., Combination therapy for the treatment of AIDS, US Appl., 19,590 (1998); Chem. Abstr., 131, 690y (1999).
10. Romero, D., Thomas, R., May, P., and Poel, T., Anti-AIDS heteroaryl-substituted piperazines, aminopiperidines and peperidines, US Appl., 354,925, 13 Dec. (1994); Chem. Abstr., 125, 142777g (1996).
11. Samia M. Rida, Soad A. M. El-Hawash, Hesham T. Y. Fahmy1, Aly A. Hazza, and Mostafa M. M. El-Meligy., Synthesis and In Vitro Evaluation of Some Novel Benzofuran Derivatives as Potential Anti-HIV-1, Anticancer, and Antimicrobial Agents, Arch Pharm Res Vol 29, No 1, 16-25, (2006).
12. James, L. T., Carly, A. P., Erik, C. M., Heather, L. H., Stephen, J. H., Richard, B. H., Phillip Bowen, J., Konstantinos, K., John, A. H., and Moses Lee, Sequence selective recognition of DNA by hairpin conjugates of a racemic seco-cyclopropaneindoline- 2-benzofurancarboxamide and polyamides. Bioorg. Med. Chem., 12, 2245-2248 (2002).
13. Vinh, T. K., Yee, S. W., Kirby, A. J., Nicholls, P. J., and Simons, C., 1-[(Benzofuran-2-yl)phenylmethyl]triazoles as steroidogenic inhibitors: Synthsis and in vitro inhibition of human placental CYP19 aromatase. Anticancer Drug Res., 16, 217- 225 (2001).
14. Wang, Y., Yuan, H., Ye, W., Wright, S., Wang, H., and Larrick, J., Synthesis and preliminary biological evaluation of CC- 1065 Analogues: Effects of different linkers and terminal amides on biological activity. J. Med. Chem., 43, 1541-1549 (2000).
15. Whomsley, R., Fernandez, E., Nicholls, P. J., Smith, H. J., Lombardi, P., and Pestellini, V., Substituted 1-[(Benzofuran- 2-yl)phenylmethyl]imidazoles as potent inhibitors of aromatase in vitro and female rats in vivo. J. Steroid Biochem. Mol. Biol., 44, 675-676 (1993).
16. Koka, M., Sevi, S., Kirilmis, C., Kazaz, C., Ozbek, B., and Otuk, G., Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 1. Synthesis and antimicrobial activity of Benzofuran-2-yl-(3-phenyl-3-methylcyclobutyl)- ketoxime derivatives. Eur. J. Med. Chem, 40, 1351-1358 (2005).
17. Wahab Khan, M., Jahangir Alam, M., Rashid, M., and Chowdhury, R., A new structural alternative in benzo[b]furans for antimicrobial activity. Bioorg. Med. Chem., 13, 4796-4805 (2005).
18. Kawasaki, K., Masubuchi, M., Morikami, K., Sogabi, S., Aoyama, Ebiike, H., Niizuma, S., Hayase, M, Fujii, T., Sakata, K., Sindoh, H., Shiratori, Y., Aoki, Y., Ohtsuka, T., and Shimma,N., Design and synthesis of novel benzofurans as antifungal agents targeting fugal N-myristoyltransferase. Part 3. Bioorg. Med. Chem. Lett., 13, 87-91 (2003).
19. A. Kleemann, J. Engel, Pharmaceutical Substances, Syntheses, Patents, Applications; Thieme, Stuttgart, New York, 2001, pp. 94, 204, 206, 208.
20. G. Bourgery, Ph. Dostert, A. Lacour, M. Langlois, B. Pourrias, J. Tisne-Versailles, J. Med. Chem., 24, 159 (1981).
21. V. Pastellini, A. Giolitti, F. Pasqui, L. Abelli, C. Cutrufo, G. De Salvia, S. Evangelista, A. Meli, Eur. J. Med. Chem., 23, 203 (1988).
22. Albert W. Burgstahler and Leonard R. Worden1Organic Syntheses, Coll. Vol. 5, p.251 (1973); Vol. 46, p.28 (1966).
23. Ahluwalia V. K., Aggarwal R., Comprehensive practical organic chemistry preparation and quantitative analysia, Universities press (India) Pvt. Ltd. Hyderabad India, 1st edition reprint 2004, pp. 124.
24. Furniss B. S., Hannaford A. J. , Smith P. W. G., Tatchell A. R., Vogel’s textbook of practical organic chemistry, Pearson education (singapore) Pvt. Ltd. Indian branch,482 FIE Patarganj Delhi India., 5th edition, reprint 2005, pp. 1160.
25. Matthew E. Dudley, M. Monzur Morshed, and M. Mahmun Hossain, Org. Synth. 2009, 86, 172-180
26. Werner R. Boehme, Organic Syntheses, Coll. Vol. 4, p.590 (1963); Vol. 33, p.43 (1953).
27. Santana L., Teijeira Ma, Uriarte E a, Teran C ., Linares B ., Villar R. , Laguna R., Cano E., AM1 theoretical study, synthesis and biological evaluation of some benzofuran analogues of anti-inflammatory arylalkanoic acids., European Journal of Pharmaceutical Sciences 7 (1998) 161 –166
28. Shen, J. X.; Zhang, P. Z.; Qiao, M. Acta. Pharm. Sinica 1988, 23, 545.
29. Yuan Chen, Shaopeng Chen, Xin Lu, Hao Cheng, Yingyong Oua, Huimin Cheng, Guo-Chun Zhou, Synthesis, discovery and preliminary SAR study of benzofuran derivatives as angiogenesis inhibitors, Bioorganic & Medicinal Chemistry Letters 19 (2009) 1851–1854.
30. Giza, C. A.; Hinman, R. L. J. Org. Chem. 1964, 29, 1453.
31. Fiandanese, V.; Bottalico, D.; Marchese, G.; Punzi, A. Tetrahedron Lett. 2003, 44, 9087-9090.
32. Kurisaki, T.; Naniwa, T.; Yamamoto, H.; Imagawa, H.; Nishizawa, M. Tetrahedron Lett. 2007, 48, 1871-1874;
33. Colobert, F.; Castanet, A.-S.; Abillard, O. Eur. J. Org. Chem. 2005, 3334-3341;
34. Zhang, H.; Larock, R. C. J. Org. Chem. 2002, 67, 7048-7056.
35. Vito Fiandanese, Daniela Bottalico, Giuseppe Marchese, Angela Punzi, A straightforward synthesis of indole and benzofuran derivatives, Tetrahedron 64 (2008) 53-60
36. Kamal M. Dawood, Hassan Abdel-Gawad, Eman A. Rageb, Ellithey M. and Hanan A. Mohamed., Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles/ Bioorg. Med. Chem. 14 (2006) 3672–3680.
37. N. Gündo?du-Karaburun, Benkli K., Tunali Y., Ümit U., Demirayak S., Synthesis and antifungal activities of some aryl [3-(imidazol-1-yl/triazol-1-ylmethyl) benzofuran-2-yl] ketoximes.,European Journal of Medicinal Chemistry 41 (2006) 651–656.
38. Zaitsev S. A. , Onuchina O. A., Alekseeva L. M., Arzamastsev A. P., Kalinkina M. A., and Granik V. G., Synthesis and antiinflammatory activity of n-[1-(5-r-oxy-2-methyl-benzofuran-3-yl)ethyl]- n-hydroxyureas., Pharmaceutical Chemistry JournalVol. 39, No. 12, (2005) 636 – 640.
39. Bakr F. Abdel-Wahab a, Hatem A. Abdel-Aziz , Essam M. Ahmed., Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3- arylbutan-1-ones and 3-(benzofuran-2 -yl)-4, 5-dihydro-5-aryl-1- [4-(aryl)-1, 3-thiazol-2-yl] -1H-pyrazoles., European Journal of Medicinal Chemistry 44 (2009) 2632–2635.
40. Hayakawa I., Shioya R., Agatsuma T., Furukawa H. and Sugano Y., Thienopyridine and benzofuran derivatives as potent anti-tumor agents possessing different structure–activity relationships., Bioorg. Med. Chem. Lett. 14 (2004) 3411–3414
41. S. Nattel, M. Talajic, B. Fermini, D. Roy, Amiodarone e pharmacology, clinical actions and relationships between them, J. Cardiovasc. Electrophysiol. 3 (1992) 266e280.
42. D. Roy, M. Talajic, P. Dorian, S. Conolly, M.J. Eisenberg, M. Green, T. Kus, J. Lambert, M. Dubuc, P. Gagne, S. Nattel, B. Thibault, Amiodarone to prevent recurrence of atrial fibrillation, N. Engl. J. Med. Chem.342 (2000) 913-920.
43. Ichiro Hayakawa, Rieko Shioya, Toshinori Agatsuma, Hidehiko Furukawa, Shunji Naruto and Yuichi Sugano A library synthesis of 4-hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as anti-tumor agents, Bioorganic & Medicinal Chemistry Letters 14 (2004) 4383–4387.
44. Okiko Miyata, Norihiko Takeda, and Takeaki Naito, Highly Effective Synthetic Methods for Substituted 2-Arylbenzofurans Using [3,3]-Sigmatropic Rearrangement: Short Syntheses of Stemofuran A and Eupomatenoid 6 Org. Lett., Vol. 6, No. 11, (2004) 1761-1763.
45. Hiroshi Morita, Tomoe Tsuchiya, Koji Kishibe, Sayaka Noya, Motoo Shiro, Yusuke Hirasawa, Antimitotic activity of lobaric acid and a new benzofuran, sakisacaulon A from Stereocaulon sasakii, Bioorganic & Medicinal Chemistry Letters 19 (2009) 3679–3681.
46. Irina N. Gaisina, Franck Gallier, Andrei V. Ougolkov, Ki H. Kim, Toru Kurome, Songpo Guo, Denise Holzle, Doris N. Luchini, Sylvie Y. Blond, Daniel D. Billadeau and Alan P. Kozikowski, From a Natural Product Lead to the Identification of Potent and Selective Benzofuran-3-yl-(indol-3-yl)maleimides as Glycogen Synthase Kinase 3 β Inhibitors That Suppress Proliferation and Survival of Pancreatic Cancer Cells, J. Med. Chem. 2009, 52, 1853–1863.
47. Gang Ni, Qing-Jian Zhang, Zhong-Fei Zheng, Ruo-Yun Chen, and De-Quan Yu, 2-Arylbenzofuran Derivatives from Morus cathayana, J. Nat. Prod. 2009, 72, 966–968.
48. Shadia A. Galal, Amira S. Abd El-All, Mohamed M. Abdallah, Hoda I. El-Diwani, Synthesis of potent antitumor and antiviral benzofuran derivatives, Bioorganic & Medicinal Chemistry Letters 19 (2009) 2420–2428.
49. Daniele Simoni, Romeo Romagnoli, Riccardo Baruchello, Riccardo Rondanin, Michele Rizzi, Maria Giovanna Pavani, Domenico Alloatti, Giuseppe Giannini, Marcella Marcellini, Teresa Riccioni, Massimo Castorina, Mario B. Guglielmi, Federica Bucci, Paolo Carminati, and Claudio Pisano, Novel Combretastatin Analogues Endowed with Antitumor Activity, J. Med. Chem. 2006, 49, 3143-3152.
50. Yuqiang Wang, Lianfa Li, Wenqing Ye, Zhiming Tian, Wei Jiang, Hong Wang, Susan C. Wright, and James W. Larrick, CC-1065 Analogues Bearing Different DNA-Binding Subunits: Synthesis, Antitumor Activity, and Preliminary Toxicity Study, J. Med. Chem. 2003, 46, 634-637.
51. Marlon Cowart, Ramin Faghih, Michael P. Curtis, Gregory A. Gfesser, Youssef L. Bennani, Lawrence A. Black, Liping Pan, Kennan C. Marsh, James P. Sullivan, Timothy A. Esbenshade, Gerard B. Fox, and Arthur A. Hancock, 4-(2-[2-(2(R)-Methylpyrrolidin-1-yl) ethyl] benzofuran-5-yl) benzonitrile and Related 2-Aminoethyl benzofuran H3 Receptor Antagonists Potently Enhance Cognition and Attention, J. Med. Chem. 2005, 48, 38-55.
52. Yu-Ming Pu, Timothy Grieme, Ashok Gupta, Daniel Plata, Ashok V. Bhatia, Marlon Cowart, and Yi-Yin Ku, A Facile and Scaleable Synthesis of ABT-239, A Benzofuranoid H3 Antagonist, Organic Process Research & Development 2005, 9, 45-50.
53. Minghua Sun, Chen Zhao, Gregory A. Gfesser, Christine Thiffault, Thomas R. Miller, Kennan Marsh, Jill Wetter, Michael Curtis, Ramin Faghih, Timothy A. Esbenshade, Arthur A. Hancock, and Marlon Cowart, Synthesis and SAR of 5-Amino- and 5-(Aminomethyl)benzofuran Histamine H3 Receptor Antagonists with Improved Potency, J. Med. Chem. 2005, 48, 6482-6490.
54. Aranapakam M. Venkatesan, O. Dos Santos, John Ellingboe, Deborah A. Evrard, Boyd L. Harrison, Deborah L. Smith, Rosemary Scerni, Geoffrey A. Hornby, Lee E. Schechter, Terrence H. Andree, Novel benzofuran derivatives with dual 5-HT1A receptor and serotonin transporter affinity, Bioorganic & Medicinal Chemistry Letters 20 (2010) 824–827.
55. Stephane Cuisiat, Nathalie Bourdiol, Vincent Lacharme, Adrian Newman-Tancredi, Francis Colpaert, and Bernard Vacher, Towards a New Generation of Potential Antipsychotic Agents Combining D2 and 5-HT1A Receptor Activities J. Med. Chem. 2007, 50, 865-876.
56. Stefano Rizzo, Ce´line Rivie`re, Lorna Piazzi, Alessandra Bisi, Silvia Gobbi, Manuela Bartolini, Vincenza Andrisano, Fabiana Morroni, Andrea Tarozzi, Jean-Pierre Monti, and Angela Rampa, Benzofuran-Based Hybrid Compounds for the Inhibition of Cholinesterase Activity, β Amyloid Aggregation, and A β Neurotoxicity, J. Med. Chem. 2008, 51, 2883–2886
57. Masahiro Ono, Hidekazu Kawashima, Akemi Nonaka, Tomoki Kawai, Mamoru Haratake, Hiroshi Mori, Mei-Ping Kung, Hank F. Kung, Hideo Saji, and Morio Nakayama, Novel Benzofuran Derivatives for PET Imaging of â-Amyloid Plaques in Alzheimer’s Disease Brains, J. Med. Chem. 2006, 49, 2725-2730
58. Stanislav A. Bakunov, Svetlana M. Bakunova, Arlene S. Bridges, Tanja Wenzler, Todd Barszcz, Karl A. Werbovetz, Reto Brun, and Richard R. Tidwell, Synthesis and Antiprotozoal Properties of Pentamidine Congeners Bearing the Benzo-furan Motif, J. Med. Chem. 2009, 52, 5763–5767.
59. Stanislav A. Bakunov, Svetlana M. Bakunova, Tanja Wenzler, Todd Barszcz, Karl A. Werbovetz, Reto Brun, and Richard R. Tidwell, Synthesis and Antiprotozoal Activity of Cationic 2-Phenylbenzofurans, J. Med. Chem. 2008, 51, 6927–6944.
60. Svetlana M. Bakunova, Stanislav A. Bakunov, Tanja Wenzler, Todd Barszcz, Karl A. Werbovetz, Reto Brun, James Edwin Hall and Richard R. Tidwell, Synthesis and in Vitro Antiprotozoal Activity of Bisbenzofuran Cations, J. Med. Chem. 2007, 50, 5807-5823.
61. Cumhur Kirilmis, Misir Ahmedzade, Su¨leyman Servi, Murat Koca, Ahmet Kizirgil, Cavit Kazaz, Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives, European Journal of Medicinal Chemistry 43 (2008) 300-308.
62. Kuntal Manna, Yadvendra K. Agrawal, Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity, Bioorganic & Medicinal Chemistry Letters 19 (2009) 2688–2692
63. Larry J. Powers, Chemistry and Antibacterial Activity of Nitrobenzofurans, Journal of Medicinal Chemistry, 1976, Val. 19, No. 1, 57-62.
64. Miyako Masubuchi, Hirosato Ebiike, Ken-ichi Kawasaki, Satoshi Sogabe, Kenji Morikami, Yasuhiko Shiratori, Shinji Tsujii, Toshihiko Fujii, Kiyoaki Sakata, Michiko Hayase, Hidetoshi Shindoh, Yuko Aoki, Tatsuo Ohtsuka, and Nobuo Shimma, Synthesis and Biological Activities of Benzofuran Antifungal Agents Targeting Fungal N-Myristoyltransferase, Bioorganic & Medicinal Chemistry 11 (2003) 4463–4478.
65. Bo Carlsson, B. N. Singh, Marcel Temciuc, Stefan Nilsson, Yi-Lin Li, Charlotta Mellin, and Johan Malm, Synthesis and Preliminary Characterization of a Novel Antiarrhythmic Compound (KB130015) with an Improved Toxicity Profile Compared with Amiodarone, J. Med. Chem. 2002, 45, 623-630.
66. Guy Bourgery, Philippe Dostert, Alain Lacour, Michel Langlois, Bernard Pourrias, and Jacky Tisne-Versailles, Synthesis and Antiarrhythmic Activity of New Benzofuran Derivatives, J. Med. Chem. 1981, 24, 159-167.
67. Xizhen Jiang 1, Wenlu Liu 1, Wei Zhang, Faqin Jiang, Zhe Gao, Hao Zhuang, Lei Fu Synthesis and antimicrobial evaluation of new benzofuran derivativesEuropean Journal of Medicinal Chemistry. 2011,46 3526-3530.
68. Basnet P, Kadota S, Terashima S, ShimizuM, Namba T.Source Two new 2-arylbenzofuran derivatives from hypoglycemic activity-bearing fractions of Morus insignis. Chem Pharm Bull (Tokyo). 1993 Jul;41(7):1238-43.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









