About Authors:
Debayan Dey*, G.D Pattnaik, Bijay kumar Sahoo
Dept.of Pharmaceutics
College of Pharmaceutical Sciences,
Puri, Orissa
INTRODUCTION
Oral administration is the most convenient and preferred means of any drug delivery to the systematic circulation. Oral controlled release drug delivery have recently been of increasing interest in pharmaceutical field to achieve improved therapeutic advantages, such as ease of dosing administration, patient compliance and flexibility in formulation. Drugs that are easily absorbed from gastrointestinal tract (GIT) and have short half-lives are eliminated quickly from the systemic circulation. Frequent dosing of these drugs is required to achieve suitable therapeutic activity.
[adsense:336x280:8701650588]
To avoid this limitation, the development of oral sustained-controlled release formulations is an attempt to release the drug slowly into the gastrointestinal tract (GIT) and maintain an effective drug concentration in the systemic circulation for a long time. After oral administration, such a drug delivery would be retained in the stomach and release the drug in a controlled manner, so that the drug could be supplied continuously to its absorption sites in the gastrointestinal tract (GIT). These drug delivery systems suffer from mainly two adversities: the short gastric retention time(GRT) and unpredictable short gastric emptying time (GET), which can result in incomplete drug release from the dosage form in the absorption zone (stomach or upper part of small intestine) leading to diminished efficacy of administered dose . To formulate a site-specific orally administered controlled release dosage form, it is desirable to achieve a prolong gastric residence time by the drug delivery. Prolonged gastric retention improves bioavailability, increases the duration of drug release, reduces drug waste, and improves the drug solubility that are less soluble in a high pH environment. Also prolonged gastric retention time (GRT) in the stomach could be advantageous for local action in the upper part of the small intestine e.g. treatment of peptic ulcer, etc. Gastro retentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper gastrointestinal tract (GIT) for local or systemic effects.
Reference ID: PHARMATUTOR-ART-1200
NEED FOR GASTRORETENTIVE DRUG DELIVERY SYSTEM
1. Drugs acting locally in the stomach
E.g. Antacids and drugs for H. Pylori viz., Misoprostol
2. Drugs that are primarily absorbed in the stomach
E.g. Amoxicillin
3. Drugs that is poorly soluble at alkaline pH
E.g. Furosemide, Diazepam, Verapamil, etc.
4. Drugs with a narrow window of absorption
E.g. Cyclosporine, Methotrexate, Levodopa, etc.
5. Drugs which are absorbed rapidly from the GI tract.
E.g. Metonidazole, tetracycline.
6. Drugs that degrade in the colon.
E.g. Ranitidine, Metformin HCl.
7. Drugs that disturb normal colonic microbes
E.g. antibiotics against Helicobacter pylori.
DRUGS THOSE ARE UNSUITABLE FOR GASTRORETENTIVE DRUG DELIVERY SYSTEMS
1.Drugs that have very limited acid solubility.
E.g. phenytoin etc.
2.Drugs that suffer instability in the gastric environment.
E.g. erythromycin etc.
3.Drugs intended for selective release in the colon.
E.g. 5- amino salicylic acid and corticosteroids etc.
Physiology of the Stomach
The GI tract is essentially a tube about nine metres long that runs through the middle of the body from the mouth to the anus and includes the throat (pharynx), oesophagus, stomach, small intestine (consisting of the duodenum, jejunum and ileum) and large intestine (consisting of the cecum, appendix, colon and rectum).
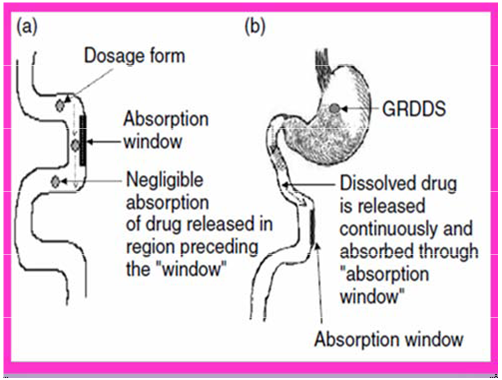
Drug absorption in the case of-
(a) Conventional dosage forms,
(b) Gastroretentive drug delivery systems.
Ø Gastric Emptying
Gastric emptying occurs during fasting as well as fed states. Gastric emptying occurs s a result of gastric contraction, the nature of which depends on the contents of the stomach. Thus gastric emptying can be conveniently classified into gastric emptying of liquid, digestible solids, and indigestible solids. Liquids empty from the stomach as a result of Intragastric pressure generated by slow muscular contractions occurring mainly from the proximal stomach (i.e. the upper body of the stomach). The removal of liquid is First order, i.e., the volume of liquid emptied per unit time is directly proportional to the volume remaining in the stomach. Digestible solids are known to be emptied only when they have been changed to a thick, creamy substance called chyme.
Indigestible solids including oral dosage forms are known to be emptied from the stomach in fasting state by a distinct cycle of Myoelectrical activity known as the Interdigestive Migrating myoelectric complex (IMMC).
[adsense:468x15:2204050025]
Gastric motility
This is called the interdigestive myloelectric cycle or migrating myloelectric cycle (MMC), which is further divided into following 4phases:-
1. Phase I (basal phase)lasts from 40 to 60 minutes with rare contractions.
2. Phase II (preburst phase)lasts for 40 to 60 minutes with intermittent action potential and contractions. As the phase progresses the intensity and frequency also increases gradually.
3. Phase III (burst phase)lasts for 4 to 6 minutes. It includes intense and regular contractions for short period. It is due to this wave that all the undigested material is swept out of the stomach down to the small intestine. It is also known as the housekeeper wave.
4. Phase IV lasts for 0 to 5 minutes and occurs between phases III and I of two consecutive cycles.
Gastrointestinal Transit Time
The residence time of liquid & solid foods in each segment of the GI tract is different. Since most drugs are absorbed from the upper intestine (duodenum, jejunum, and ileum), the total effective time for drug absorption is 3-8 hrs.This is why one has to take most drugs 3-6 times a day.
|
Segment |
Type of food |
|
|
Solid
|
Liquid |
|
|
Stomach |
10-30mins |
1-3hrs |
|
Duodenum |
?60secs |
?60secs |
|
Jejunum & ileum |
3hr±1.5hr |
4hr±105hr |
|
Colon |
- |
20-50hr |
FACTORS CONTROLLING GASTRIC RETENTION OF DOSAGE FORMS
Density of dosage forms
A density of <1.0 gm/cm3 is required to exhibit floating property.
Shape and size of the dosage form
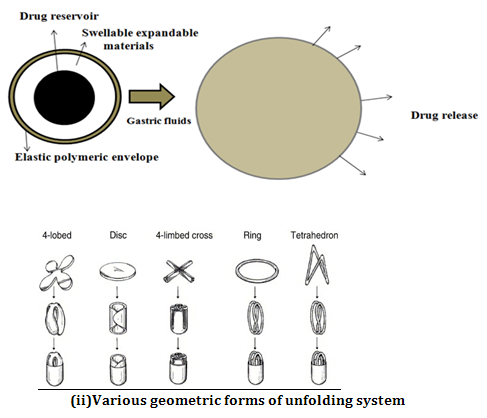
Dosage forms having a diameter of more than 7.5 mm show a better gastric residence time compared with one having 9.9 mm. Ring-shaped and tetrahedron-shaped devices have a better gastric residence time as compared with other shapes.
Food intake and its nature
The presence or absence of food in the gastrointestinal tract (GIT) influences the gastric retention time (GRT) of the dosage form. Usually the presence of food in the gastrointestinal tract (GIT) improves the gastric retention time (GRT) of the dosage form and thus, the drugs absorption increases by allowing its stay at the absorption site for a longer period. Again, increase in acidity and caloric value shows down gastric emptying time (GET), which can improve the gastric retention of dosage forms.
Fed or unfed state
Under fasting conditions: GI motility is characterized by periods of strong motor activity or the migrating myoelectric complex (MMC) that occurs every 1.5 to 2 hours. The MMC sweeps undigested material from the stomach and, if the timing of administration of the formulation coincides with that of the MMC, the GRT of the unit can be expected to be very short. However, in the fed state, MMC is delayed and GRT is considerably longer.
Nature of meal
Feeding of indigestible polymers or fatty acid salts can change the motility pattern of the stomach to a fed state, thus decreasing the gastric emptying rate and prolonging drug release.
Disease state
Gastric ulcer, diabetes, hypothyroidism increase GRT. Hyperthyroidism, duodenal ulcers decrease GRT.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Approaches
The strategies for delaying drug transit time through the GIT fall into one of three categories:-
Ø FLOATING – A LOW DENSITY APPROACH
Floating drug delivery systems (FDDS) or hydro-dynamically balanced systems
have a bulk density lower than gastric fluids and thus remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents, the drug is released slowly at a desired rate from the stomach. After the release of the drug, the residual system is emptied from the stomach.

Effervescent systems
These are matrix type of systems prepared with the help of swellable polymers such as Methylcellulose and chitosan and various effervescent compounds, e.g. sodium bicarbonate, tartaric acid and citric acid. They are formulated in such a way that when in contact with the gastric contents, CO2 is liberated and gets entrapped in swollen hydrocolloids, which provides buoyancy to the dosage forms.
1. Volatile liquid containing systems
These have an inflatable chamber which contains a liquid e.g. ether, cyclopentane, that gasifies at body temperature to cause the inflation of the chamber in the stomach.
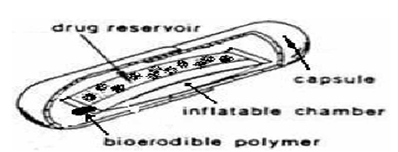
Inflation chamber
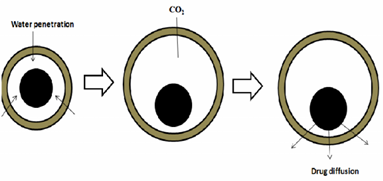
2.Gas generating systems:
These buoyant systems utilised matrices prepared with swellable polymers like methocel, polysaccharides like chitosan, effervescent components like sodium bicarbonate, citric acid and tartaric acid or chambers containing a liquid that gasifies at body temperature.The common approach for preparing these systems involves resin beads loaded with bicarbonate and coated with ethylcellulose. The coating, which is insoluble but permeable, allows permeation of water. Thus, carbon dioxide is released, causing the beads to float in the stomach.
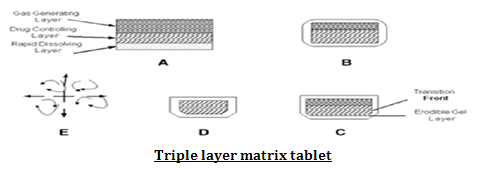
3.Matrix Tablets
Single layer matrix tablet is prepared by incorporating bicarbonates in matrix forming hydrocolloid gelling agent like HPMC, chitosan, alginate or other polymers and drug. Bilayer tablet can also be prepared by gas generating matrix in one layer and second layer with drug for its SR effect. Floating capsules also prepared by incorporating such mixtures.
Ø Noneffervescent system
Hydrodynamically balanced systems
These systems contains drug with gel-forming hydrocolloids meant to remain buoyant on the stomach content. These are single-unit dosage form, containing one or more gel-forming hydrophilic polymers. Hydroxypropyl methylcellulose (HPMC), hydroxethyl cellulose (HEC), hydroxypropyl cellulose (HPC), sodium carboxymethyl cellulose (NaCMC), polycarbophil, polyacrylate, polystyrene, agar, carrageenans or alginic acid are commonly used excipients to develop these systems. The polymer is mixed with drugs and usually administered in hydrodynamically balanced system capsule. The capsule shell dissolves in contact with water and mixture swells to form a gelatinous barrier, which imparts buoyancy to dosage form in gastric juice for a long period.
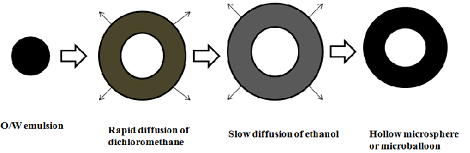
Ø Microballoons / Hollow microspheres:
Microballoons / hollow microspheres loaded with drugs in their other polymer shelf were prepared by simple solvent evaporation or solvent diffusion evaporation methods to prolong the gastric retention time (GRT) of the dosage form. Commonly used polymers to develop these systems are polycarbonate, cellulose acetate, calcium alginate, Eudragit S, agar and low methoxylated pectin etc.
1. Alginate beads
They were made by using Ca2+ and low methoxylated pectin (anionic polysaccharide) or Ca2+ low methoxylated pectin and sodium alginate. In this approach, generally sodium alginate solution is dropped into aqueous solution of calcium chloride and causes the precipitation of calcium alginate. These beads are then separated and dried by air convection and freeze drying, leading to the formulation of a porous system, which can maintain a floating force for over 12 hrs. These beads improve gastric etention time (GRT) more than 5.5 hrs.
2. Microporous compartment system:
This approach is based on the principle of the encapsulation of a drug reservoir inside a microporous compartment with pores along its top and bottom walls. The peripheral walls of the device were completely sealed to present any direct contact of the gastric surface with the undissolved drug. In the stomach the floatation chamber containing entrapped air causes the delivery system to float in the gastric fluid. Gastric fluid enters through the aperture, dissolves the drug and causes the dissolved drug for continuous transport across the intestine for drug absorption.
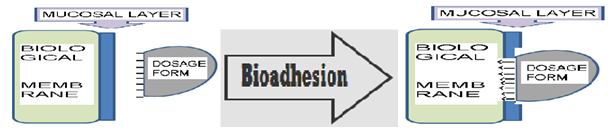
Ø BIOADHESIVE SYSTEMS
Bio/mucoadhesive systems are those which bind to the gastric epithelial cell surface or mucin and serve as a potential means of extending the Gastro retention of drug delivery system (DDS) in the stomach by increasing the intimacy and duration of contact of drug with the biological membrane.
Ø SWELLING OR EXPANDING SYSTEM
After being swallowed, these dosage forms swell to a size that prevents their passage through the pylorus. As a result, the dosage form is retained in the stomach for a long period of time. These systems are sometimes referred to as plugtypesystemsbecause they tend to remain lodged at the pyloric sphincter. As dosage form coming in contact with gastric fluid, the polymer imbibes water and swells.
The expansion can be achieved by-
i) Swelling system
ii) Unfolding system
Ø HIGH DENSITY SYSTEMS
These systems with a density of about 3 g/cm3 are retained in the antrum part of the stomach and are capable of withstanding its peristaltic movements. The only major drawbacks with such systems is that it is technically difficult to manufacture such formulations with high amount of drug (>50%) and to achieve a density of about 2.8 g/cm3. It is necessary to use diluents like barium sulfate, zinc oxide, titanium dioxide, iron powder etc. to manufacture such high density formulations.
Ø RAFT FORMING SYSTEMS
Usually, the system contains a gel forming agent and alkaline bicarbonates or carbonates responsible for the formation of CO2 to make the system less dense and float on the gastric fluids. The system contains a gel forming agent (e.g. alginic acid), sodium bicarbonate and acid neutralizer, which forms a foaming sodium alginate gel (raft) when in contact with gastric fluids. The raft thus formed floats on the gastric fluids and prevents the reflux of the gastric contents (i.e. gastric acid) into the esophagus by acting as a barrier between the stomach and esophagus.
Ø Super porous hydrogel systems
These swellable systems differ sufficiently from the conventional types to warrant separate classification. In this approach to improve gastric retention time (GRT) super porous hydrogels of average pore size >100 micro miter, swell to equilibrium size within a minute due to rapid water uptake by capillary wetting through numerous interconnected open pores. They swell to a large size and are intended to have sufficient mechanical strength to withstand pressure by gastric contraction. This is advised by co-formulation of hydrophilic particulate material.
Ø MAGNETIC SYSTEMS
This system is based on a simple idea that the dosage form contains a small internal magnet and a magnet placed on the abdomen over the position of the stomach. Ito et al. used this technique in rabbits with bioadhesives granules containing ultrafine ferrite (g-Fe2O3). They guided them to the esophagus with an external magnet (1700 G) for the initial 2 min and almost all the granules were retained in the region after 2 h10.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
EVALUATION OF GASTRORETENTIVE DOSAGEFORM
A) IN-VITRO EVALUATION
i) Floating systems
a) Buoyancy Lag Time:-
It is determined in order to assess the time takenby the dosage form to float on the top of the dissolution medium, after it is placed in the medium. These parameters can be measured as a part of the dissolution test.
b) Floating Time:-Test for buoyancy is usually performed in SGF-Simulated Gastric Fluid maintained at 370C. The time for which the dosage form continuously floats on the dissolution media is termed as floating time.
c) Specific Gravity / Density
Density can be determined by the displacement method using Benzene as displacement medium.
d) Resultant Weight:-
F = Fbuoy - FgravF = Df g V – Ds g V F = (Df – Ds) g V
F = (Df – M/V) g V
Where, F = resultant weight of object ,Df = Density of Fluid
DS = Density of Solid object
g = Gravitational force
M = Mass of dosage form
V = Volume of dosage form
e)Drug release:-
Dissolution tests are performed using the dissolution apparatus. Samples are withdrawn periodically from the dissolution medium with replacement and then analyzed for their drug content after an appropriate dilution.
f)Drug loading, drug entrapment efficiency, particle size analysis, surface characterization (for floating microspheres and beads):-
Drug loading is assessed by crushing accurately weighed sample of beads or microspheres in a mortar and added to the appropriate dissolution medium which is then centrifuged, filtered and analyzed by various analytical methods like spectrophotometry. The particle size and the size distribution of beads or microspheres is determined in the dry state using the optical microscopy method.The external and cross-sectional morphology(surface characterization) is done by scanning electron microscope (SEM).
ii) Swelling systems
a) Swelling Index
After immersion of swelling dosage form into SGF at 370C, dosage form is removed out at regular interval and dimensional changes are measured in terms of increase in tablet thickness / diameter with time.
b) Water Uptake
It is also termed asWeight Gain.
Water uptake = WU = (Wt – Wo) * 100 / Wo
Where, Wt = weight of dosage form at time t
Wo = initial weight of dosage form.
B) IN-VITRO DISSOLUTION TESTS
In vitro dissolution test is generally done by using USP apparatus with paddle and GRDDS is placed normally as for other conventional tablets. various types of modification in dissolution assembly made are as follows.
1. To prevent sticking at vessel or paddle and to improve movement of dosage form, method suggested is to keep paddle at surface and not too deep inside dissolution medium.
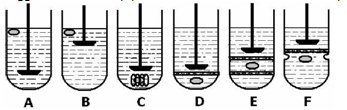
2. Floating unit can be made fully submerged, by attaching some small, loose, non- reacting material, such as few turns of wire helix, around dosage form.
3. Other modification is to make floating unit fully submerged under ring or mesh assembly and paddle is just over ring that gives better force for movement of unit.
C)IN-VIVO EVALUATION
a) Radiology
X-ray is widely used for examination of internal body systems. Barium Sulphate is widely used Radio Opaque Marker. So, BaSO4 is incorporated inside dosage form and X-ray images are taken at various intervals to view GR.
b)X-Ray/Gamma Scintigraphy X-Ray/Gamma Scintigraphy is a very popularily used evaluation parameter for floating dosage form these days. It helps to locate dosage form in the GIT and by which one can predict and correlate the gastric emptying time and the passage of dosage form in the GIT.
c) Gastroscopy
Gastroscopy is peroral endoscopy used with fiber optics or video systems. Gastroscopy is used to inspect visually the effect of prolongation in stomach. It can also give the detailed evaluation of GRDDS.
d) Magnetic Marker Monitoring
In this technique, dosage form is magnetically marked with incorporating iron powder inside, and images can be taken by very sensitive bio-magnetic measurement equipment. Advantage of this method is that it is radiation less and so not hazardous.
e) Ultrasonography
Used sometimes, not used generally because it is not traceable at intestine.
f) 13C Octanoic Acid Breath Test
13C Octanoic acid is incorporated into GRDDS. In stomach due to chemical reaction, octanoic acid liberates CO2 gas which comes out in breath. The important Carbon atom which will come in CO2 is replaced with 13C isotope. So time up to which 13CO2 gas is observed in breath can be considered as gastric retention time of dosage form. As the dosage form moves to intestine, there is no reaction and no CO2 release. So this method is cheaper than other.
Ø ADVANTAGES OF GASTRORETENTIVE DELIVERY SYSTEMS
1) Improvement of bioavailability and therapeutic efficacy of the drugs and possible reduction of dose e.g. Furosemide
2) Maintenance of constant therapeutic levels over a prolonged period and thus reduction in fluctuation in therapeutic levels minimizing the risk of resistance especially in case of antibiotics. E.g. b-lactam antibiotics (penicillins and cephalosporins)
3) For drugs with relatively short half life, sustained release may result in a flip- flop pharmacokinetics and also enable reduced frequency of dosing with improved patient Compliance.
4) They also have an advantage over their conventional system as it can be used to overcome the adversities of the gastric retention time (GRT) as well as the gastric emptying time (GET).
5) Gastro retentive drug delivery can produce prolongs and sustains release of drugs from dosage forms which avail local therapy in the stomach and small
intestine. Hence they are useful in the treatment of disorders related to stomach and small intestine.
6) The controlled, slow delivery of drug form gastro retentive dosage form provides sufficient local action at the diseased site, thus minimizing or eliminating systemic exposure of drugs. This site-specific drug delivery reduces undesirable Effects of side effects.
7) Gastro retentive dosage forms minimize the fluctuation of drug concentrations and effects. Therefore, concentration dependent adverse effects that are associated with peak concentrations can be presented. This feature is of special importance for drug with a narrow therapeutic index.
8) Gastro retentive drug delivery can minimize the counter activity of the body leading to higher drug efficiency.
Drugs Used In the Formulations of Stomach Specific
Ø Floating microspheres: Aspirin, Griseofulvin, P-nitroaniline, Ibuprofen, Ketoprofen, Piroxicam, Verapamil, Cholestyramine, Theophylline, Nifedipine, Nicardipine, Dipyridamol, Tranilast and Terfinadine.
Ø Floating granules: Diclofenac sodium, Indomethacin and Prednisolone.
Ø Films: Cinnarizine and Albendazole.
Ø Floating tablets and pills: Acetaminophen, Acetylsalicylic acid, Ampicillin, Amoxycillin trihydrate, Atenolol, Fluorouracil, Cephalexin.
Ø Floating Capsules: Chlordiazepoxide hydrogen chloride, Diazepam, Furosemide, Misoprostol, L-Dopa, Benserazide, Ursodeoxycholic acid and Pepstatin and Propranolol.
Future Prospects
While the control of drug release profiles has been a major aim of pharmaceutical research and development in the past two decades, the control of GI transit profiles could be the focus of the next two decades and might result in the availability of new products with new therapeutic possibilities and substantial benefits for patients.
Marketed Preparations
|
Product |
Active Ingredient |
Remarks |
|
Madopar |
Levodopa (100mg) Benserazide (25mg) |
Floating, CR capsules |
|
valrelease |
Diazepam (15mg) |
Floating capsules |
|
Topalkan |
Aluminum magnesium antacid |
Floating liquid alginate preparation |
|
Almagate FloatCoat |
Antacid |
Floating dosage form |
|
Liquid Gaviscon |
Alginic acid and sodium bicarbonate |
Effervescent floating liquid alginate preparation |
|
Conviron |
Ferrous sulphate |
Colloidal gel forming FDDS |
|
Colloidal gel forming FDDS |
Ciprofloxacin (1g) |
Gas generating floating form |
|
Cytotec |
Misoprostol (100mcg/200mcg) |
Bilayer floating capsule |
CONCLUSION
Based on the literature surveyed, it may be concluded that gastroretentive drug delivery offers various potential advantages for drug with poor bioavailability due their absorption is restricted to the upper gastrointestinal tract (GIT) and they can be delivered efficiently thereby maximizing their absorption and enhancing absolute bioavailability. Due to complexity of pharmacokinetics and pharmacodynamics parameters, in vivo studies are required to establish the optional dosage form for a specific drug. Another promising area of research for gastroretentive drug delivery system is eradication of Helicobacter pylori, which is now believed to be causative bacterium of chronic gastritis and peptic ulcers.
REFERENCES
1. Streubel A, Siepmann J, Bodmeier R. Gastroretentive drug delivery system. Expert Opin Drug Deliv 2006; 3(2): 217- 33.
2. Iannucelli V, Coppi G, Bernabei MT, Camerorni R. Air compertment multiple-unit system for prolonged gastric residence. Part-I. Formulation study. Int J Pharm 1998;174: 47-54.
3. Garg R, Gupta GD. Progress in controlled gastroretentive delivery systems. Trop. J Pharm Res 2008; 7(3): 1055-66.
4. Rouge N, Allemann E, Gex-Fabry M, Balant L, Cole ET, Buri P, Doelker E. Comparative pharmacokinetic study of a floating multiple-unit capsule, a high density multipleunit capsule and an immediate-release tablet containing 25 mg atenolol. Pharm Acta Helbetiae 1998; 73: 81-7.
5. Streubel A, Siepmann J, Bodmeier R. Multiple unit Gastroretentive drug delivery: a new preparation method for low density microparticles. J Microencapsul 2003; 20: 329-47.
6. Goole J, Vanderbist F, Aruighi K. Development and evaluation of new multiple-unit levodopa sustained-release floating dosage forms. Int J Pharm 2007; 334: 35-41.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









